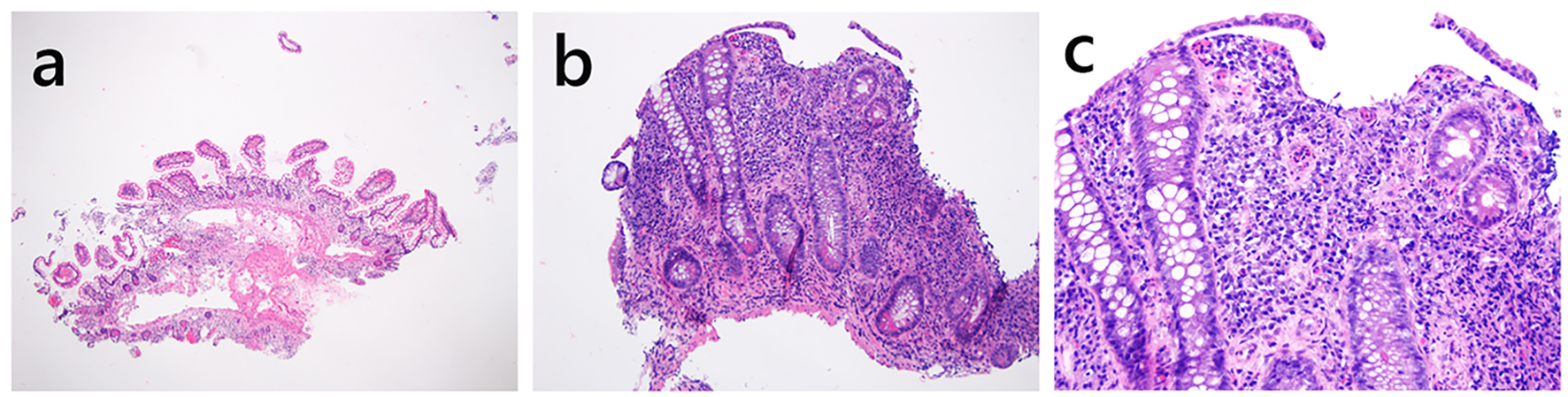
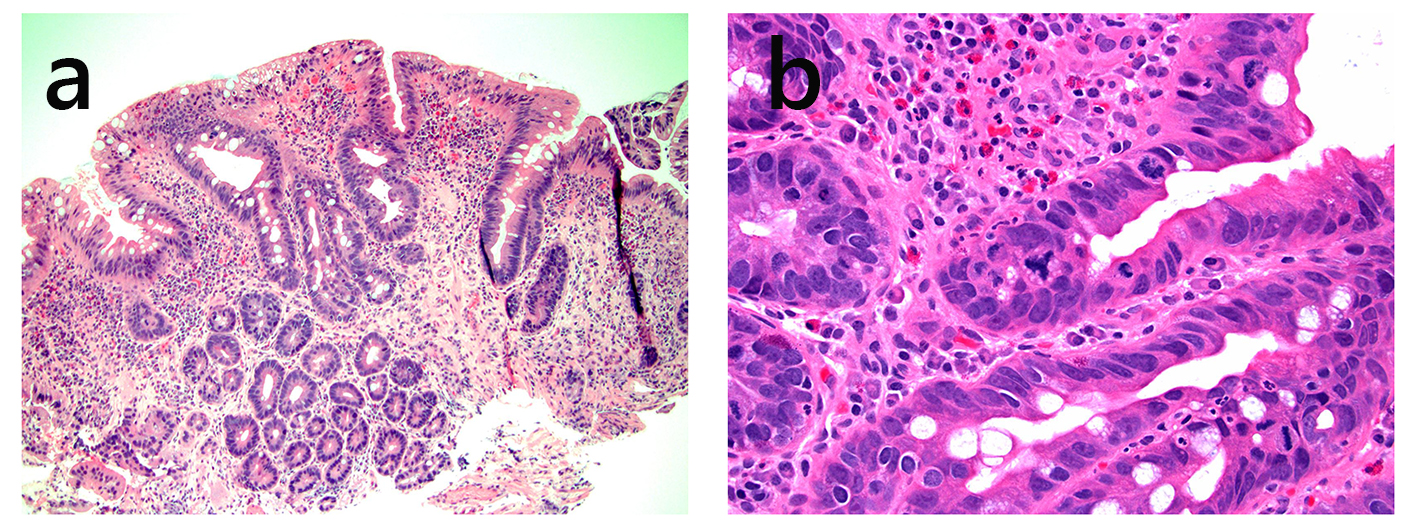
| Pouch inflammation of no clinical significance | N/A | Should not be treated with antibiotics | +/- | Variable degree of acute and chronic inflammation | +/- | Asymptomatic |
| Acute pouchitis | Acute onset, < 4 weeks symptom duration | Antibiotic- response | Variably involved | Acute inflammation, crypt abscess, chronic inflammation (+/-) | +/- | |
| Chronic pouchitis | > 4 weeks symptom duration and/or > 3 attacks in a 12-month period | Antibiotic- response, antibiotic- dependent, or antibiotic-refractory | +/- | Acute inflammation, crypt abscess, chronic inflammation, PGM (+/-) | +/- | |
| Secondary infectious pouchitis | Variable | Antibiotic- refractory* | +/- | Acute inflammation, crypt abscess, chronic inflammation (+/-), PGM (+/-), granulomatous inflammation (in some fungal infection) | +/- | Viral inclusion, fungal organisms on special stain, or positive stool C. difficile toxin results |
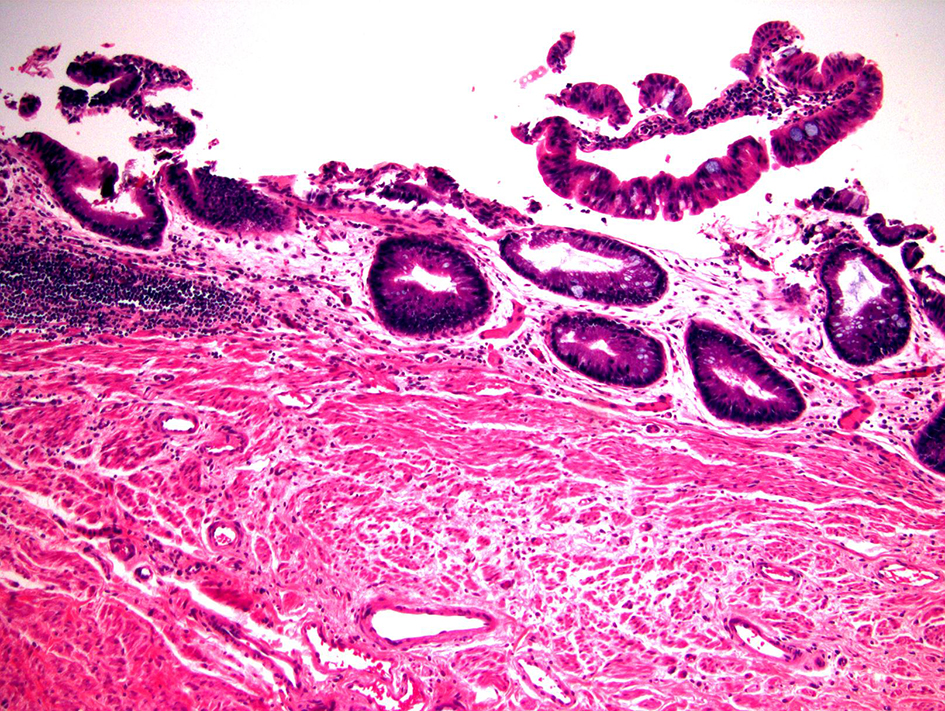
| Ischemic pouchitis | Variable | Antibiotic- refractory | Relatively normal | Acute inflammation, crypt abscess, chronic inflammation (+/-) , PGM (+/-), variable fibrosis | Relatively normal | Asymmetric and well demarcated inflammation of the pouch by endoscopy, hematoidin or hemosiderin deposits |
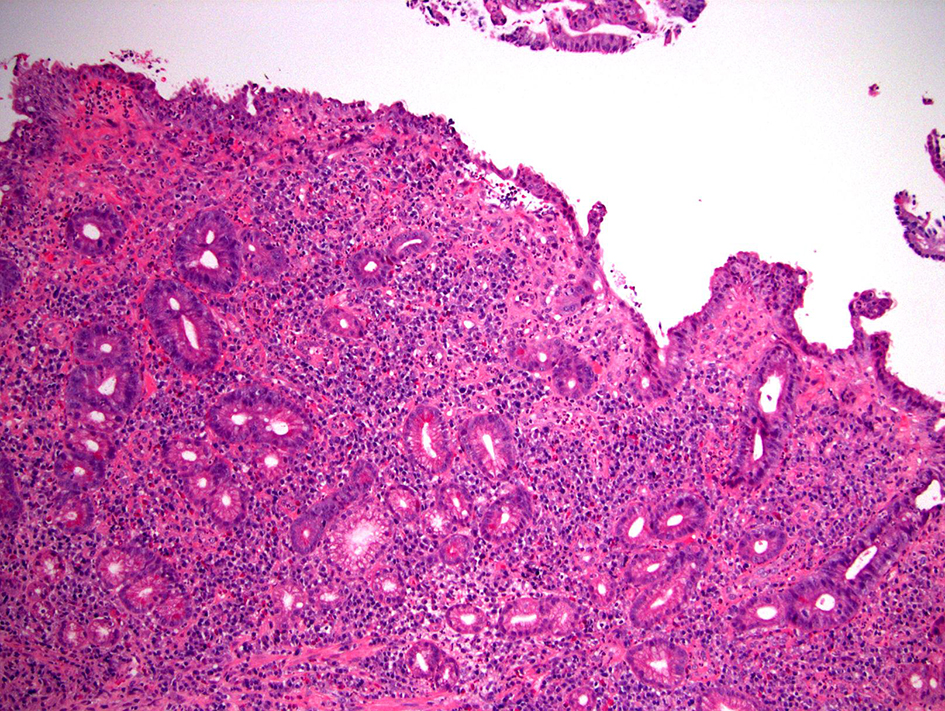
| Autoimmune pouchitis | Variable | Antibiotic- refractory | +/- | Acute inflammation, crypt abscess, chronic inflammation, villous blunting, PGM (commonly present) | +/- | Prominent deep crypt apoptosis |
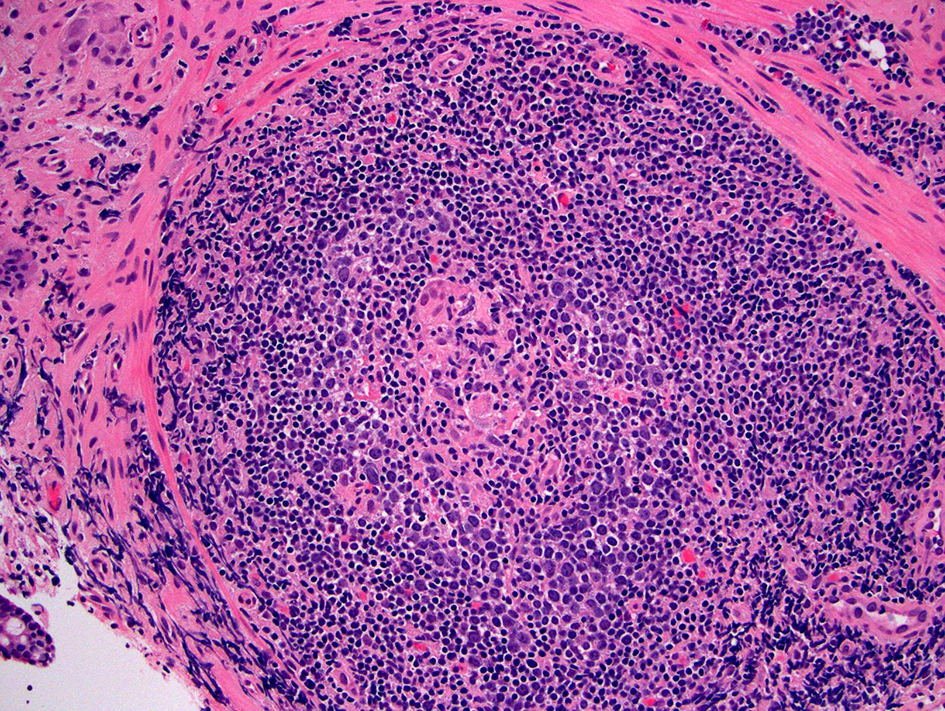
| Crohn’s disease of the pouch | Variable | Antibiotic- refractory | Variably involved | Acute and chronic inflammation, crypt abscess, villous blunting, PGM (common), non-caseating granuloma (10-12% cases) | Variably involved | Stricture or ulceration away from staple lines, and/or fistula occurring ≥ 3 months after ileostomy reversal, involvement of upper gastrointestinal tract |
| Idiopathic pre-pouch ileitis | Variable | Antibiotic- refractory | Acute and chronic inflammation, villous blunting, PGM (common) | Normal | Normal | |
| Cuffitis | Variable | Antibiotic- refractory | Relatively normal | Relatively normal | Chronic active colitis pattern | |