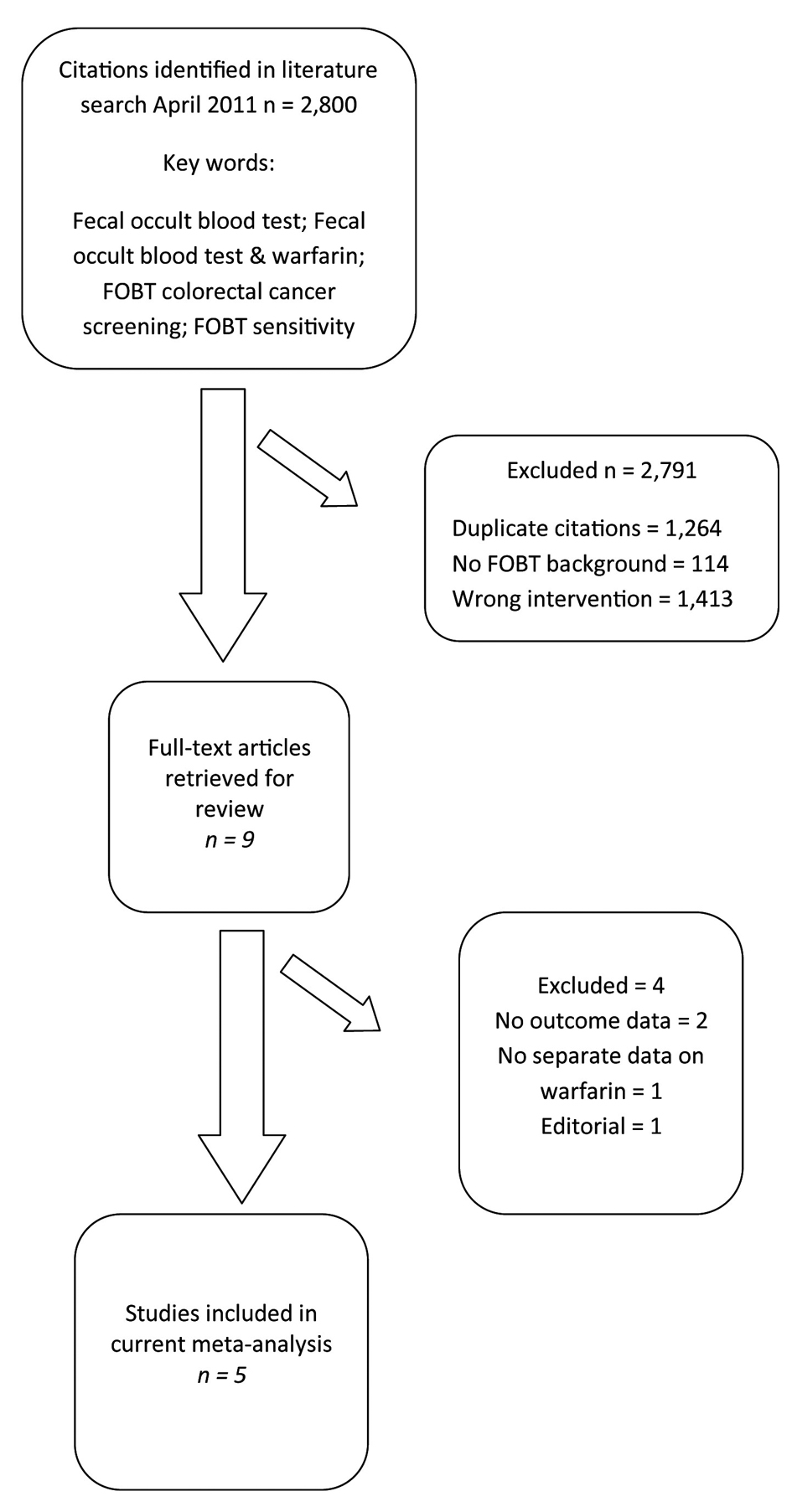
Figure 1. Selection of studies for inclusion in the meta-analysis of warfarin use during fecal occult blood testing.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Original Article
Volume 5, Number 2, April 2012, pages 45-51
Warfarin Use During Fecal Occult Blood Testing: A Meta-Analysis
Figures

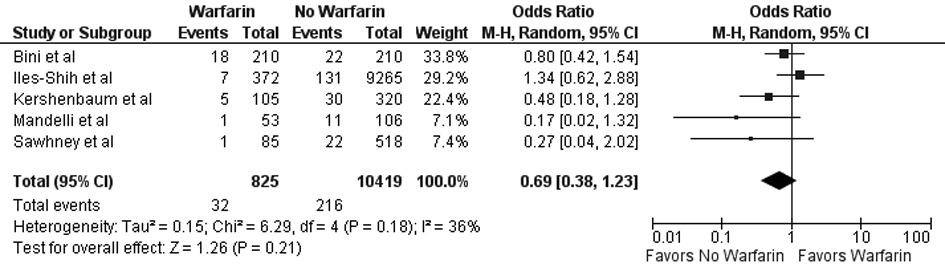
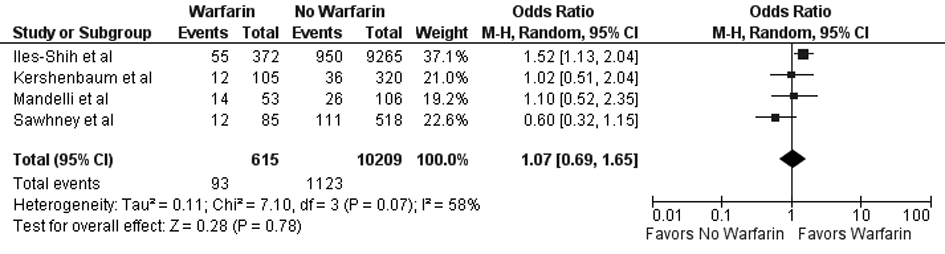
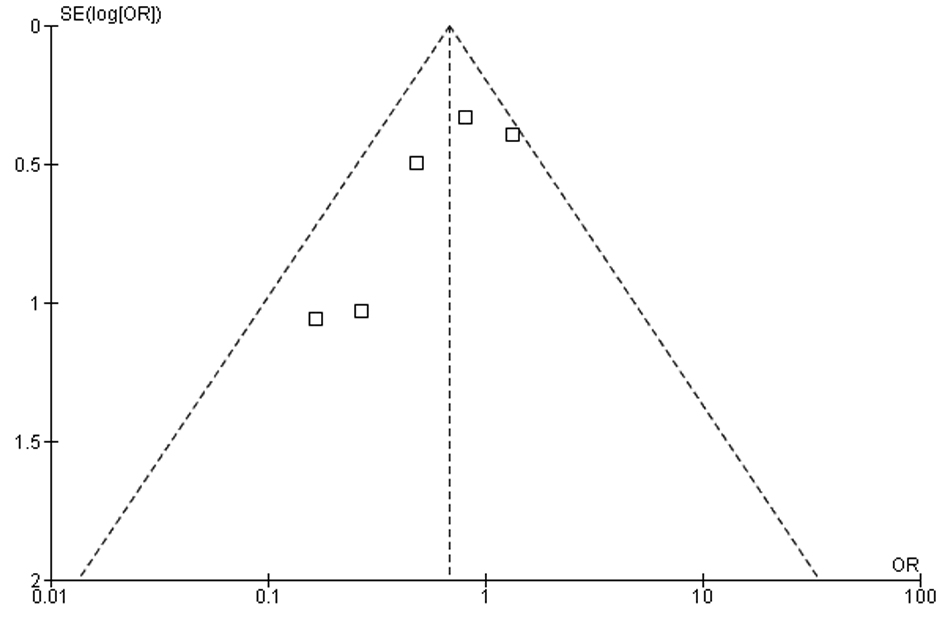
Tables
| Author | Study | Blinded | Location | FOBT | Patients (n) | Study Quality ★ |
|---|---|---|---|---|---|---|
| ★ Stars based upon Newcastle-Ottawa quality assessment scale for cohort studies (0 stars = poor, 9 stars = excellent) | ||||||
| Bini et al - 2005 | Cohort | None | United States | Hemoccult II | 420 | ★★★★★★★★★ |
| Iles-Shih et al - 2010 | Cohort | None | United States | Hemoccult II | 9637 | ★★★★★★★★★ |
| Kershenbaum et al - 2010 | Cohort | None | Israel | Hemoccult Sensa | 425 | ★★★★★★★★ |
| Sawhney et al - 2010 | Cohort | None | United States | Hemoccult II or equivalent | 603 | ★★★★★★★★ |
| Mandelli et al - 2010 | Cohort | None | Italy | iFOBT | 159 | ★★★★★★★★ |
| Outcome | Odds Ratio | 95% Confidence Interval | P-Value | I2 |
|---|---|---|---|---|
| * Random effects model and an elimination analysis was performed given statistically significant heterogeneity. | ||||
| Colonoscopic Findings | 0.88 | 0.48 - 1.62 | 0.67 | 81%* |
| Neoplasia | 0.88 | 0.58 - 1.35 | 0.57 | 76%* |
| Any Adenoma | 1.08 | 0.73 - 1.58 | 0.71 | 23% |