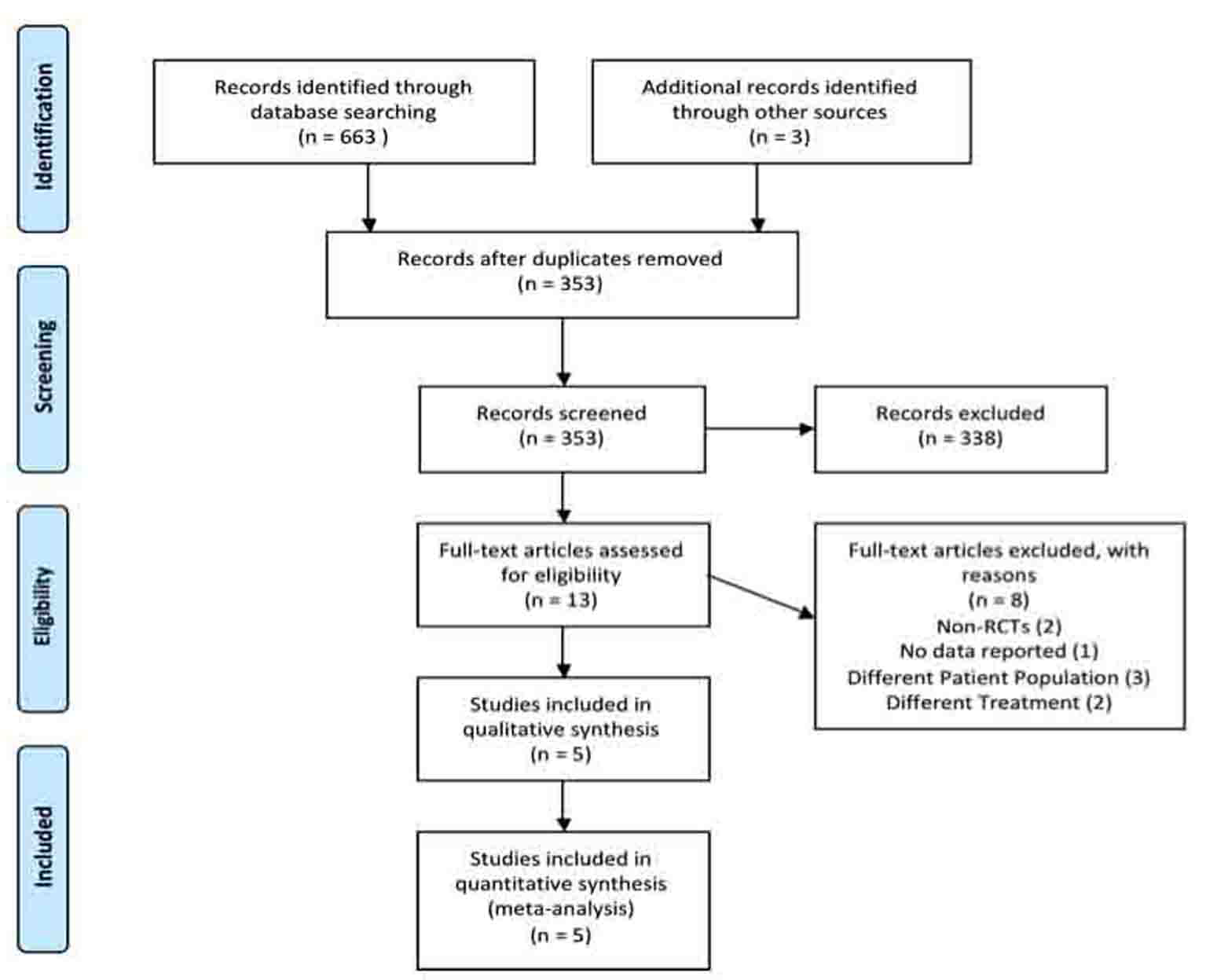
Figure 1. Systematic review PRISMA flow diagram.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Original Article
Volume 4, Number 3, June 2011, pages 97-106
Povidone-Iodine Irrigation of Subcutaneous Tissues May Decrease Surgical Site Infections in Elective Colorectal Operations: A Systematic Review
Figures

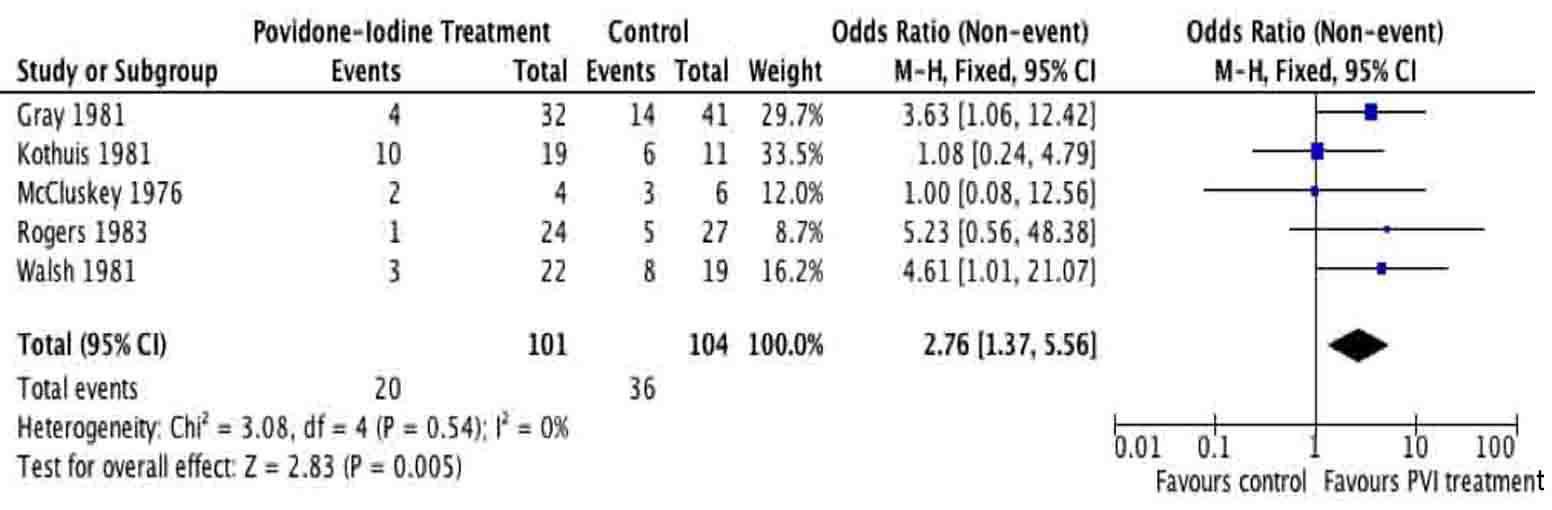
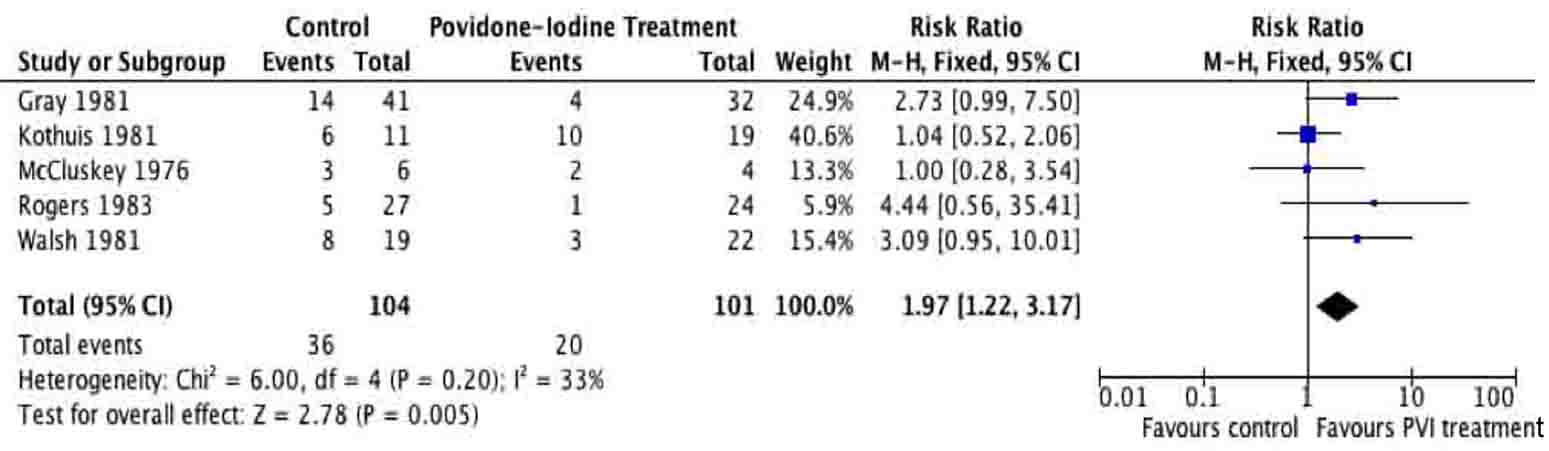
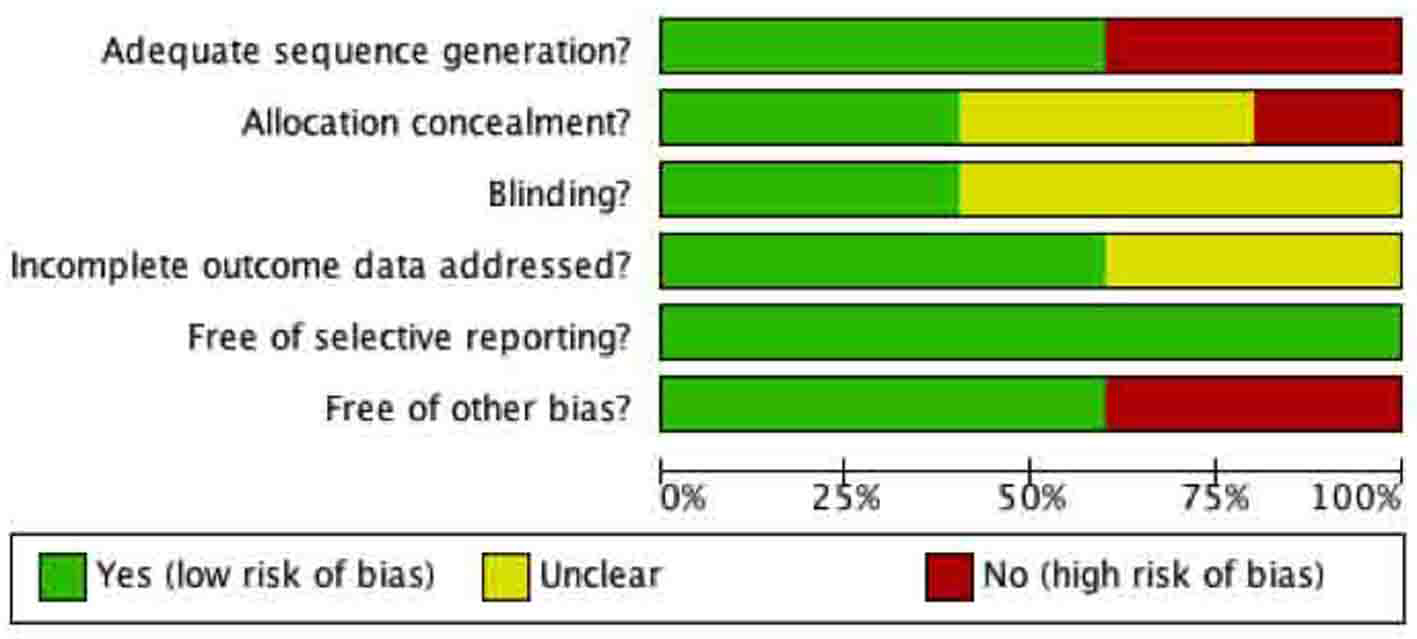
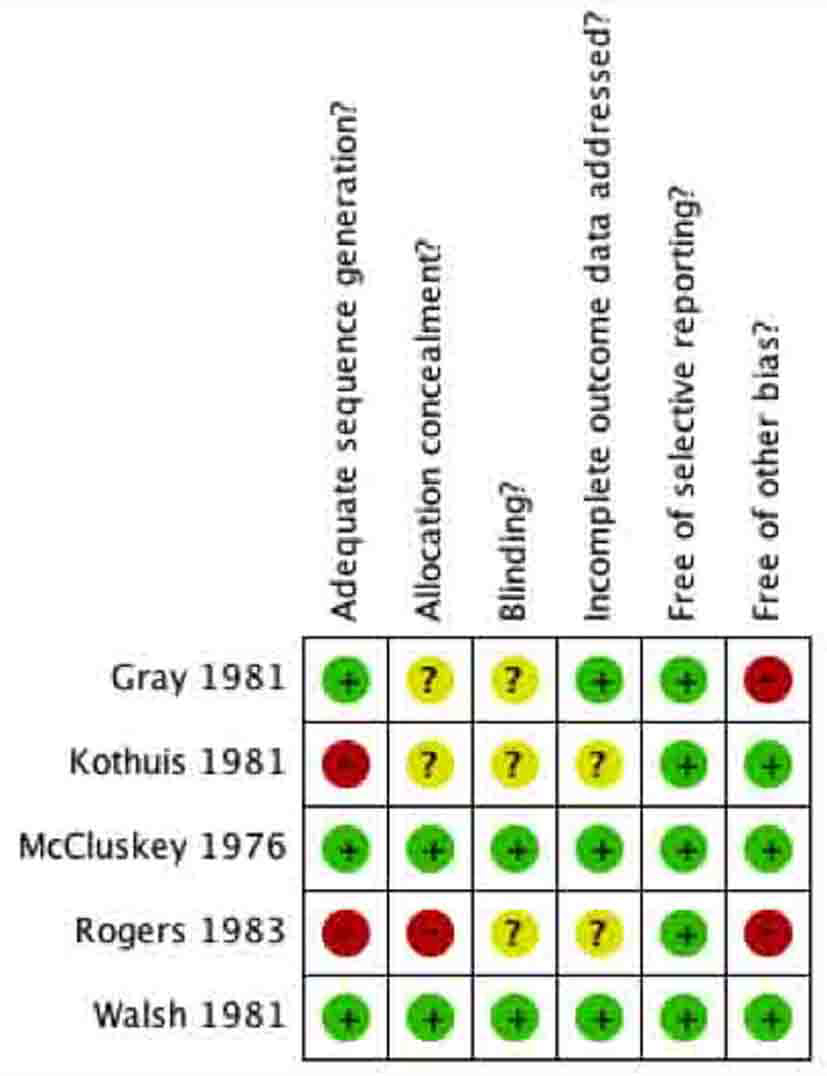
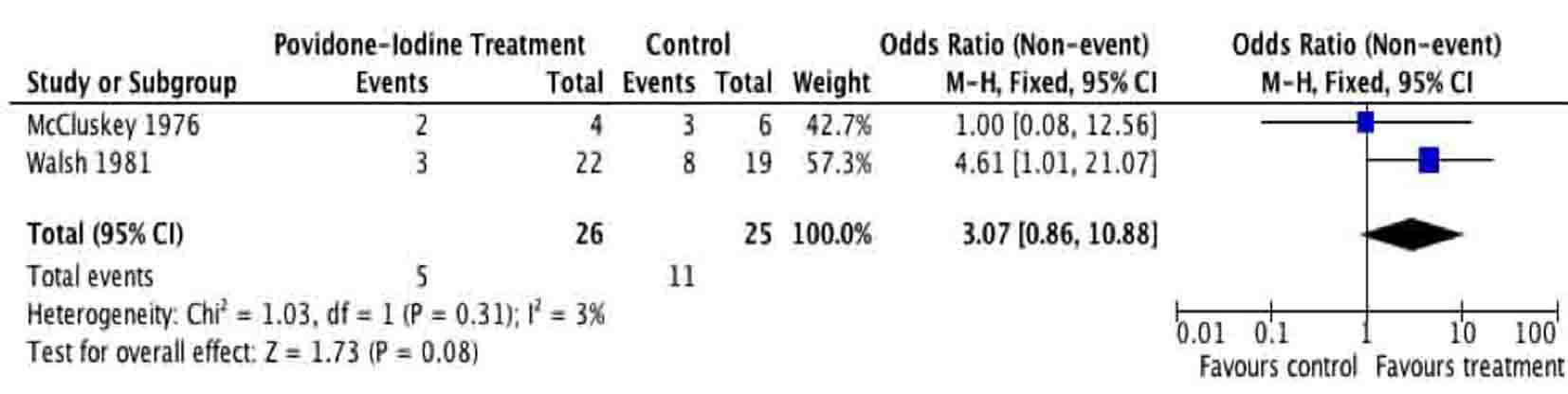
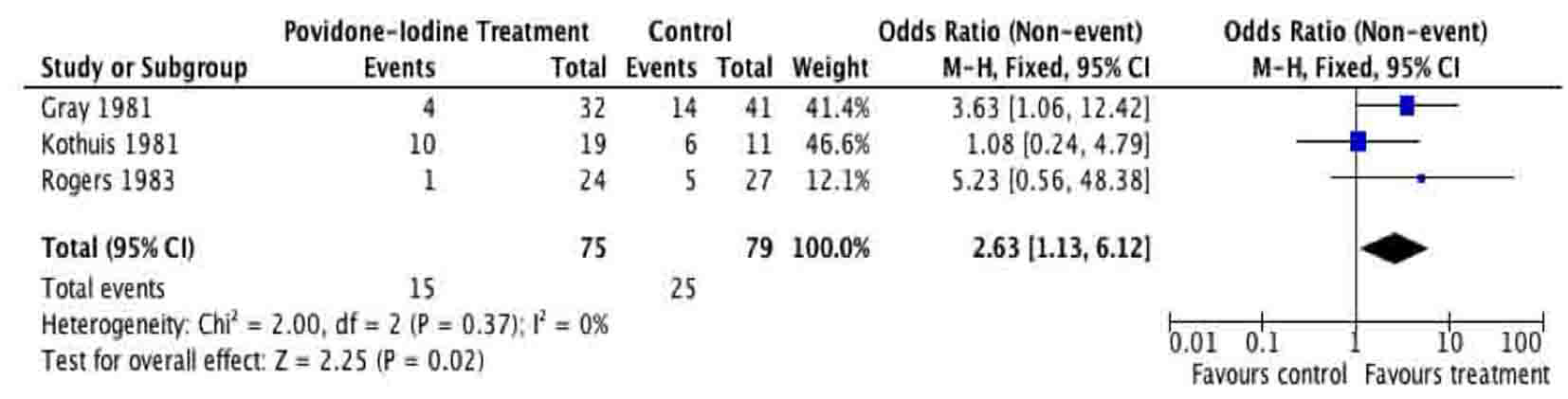
Tables
| Author, Year of Study | Design, Duration | Treatment | Control | Participants | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PVI | Controls | |||||||||
| + | Total | % | + | Total | % | |||||
| Note: RCT = Randomized Control Trial; + = Number of wound infections; PVI = Povidone-Iodine | ||||||||||
| Gray, 1981 | RCT, 15 months | PVI spray | Not sprayed | 4 | 32 | 12 | 14 | 41 | 34 | Incidence of wound infection |
| Kothuis, 1981 | RCT, ? | 10% PVI solution | Iodine tincture | 10 | 19 | 47 | 6 | 11 | 45 | Wound healing |
| McCluskey, 1976 | RCT, ? | 10% PVI solution | No solution | 2 | 4 | 50 | 3 | 6 | 50 | Incidence of infected wounds |
| Rogers, 1983 | RCT, 6 months | 10% PVI solution | Normal saline | 1 | 24 | 4.2 | 5 | 27 | 18.5 | Wound infection rate |
| Walsh, 1981 | RCT, 12 months | 5% PVI spray | Not sprayed | 3 | 22 | 14 | 8 | 19 | 42 | (1) Incidence of wound infection (2) Mean postoperative hospital stay |
| Totals | 5 RCTs | 3 solution; 2 spray | 3 non-Rx | 17 | 101 | - | 36 | 104 | - | Variable |
| Author, Year of Study | Total number of patients | # of patients included in SR | Age (years) | Gender | Operative time (min) | Preoperative antibiotics (# of patients) | Postoperative antibiotics (# of patients) | |
|---|---|---|---|---|---|---|---|---|
| M | F | |||||||
| Note: SR = Systematic Review; M = Males; F = Females; min = minutes; N/A = not available | ||||||||
| Gray, 1981 | 153 | 73 | 16 - 76 | 69 | 84 | 20 - 105 | 44 | 36 |
| Kothuis, 1981 | 220 | 30 | 48 (mean) | 132 | 88 | < 60 - 240 | ||
| McCluskey, 1976 | 110 | 10 | 40 - 65 | 46 | 64 | N/A | ||
| Rogers, 1983 | 187 | 51 | 60.2 (mean) | |||||
| Walsh, 1981 | 627 | 41 | 43.4 (mean) | 314 | 313 | 139 | 83 | |