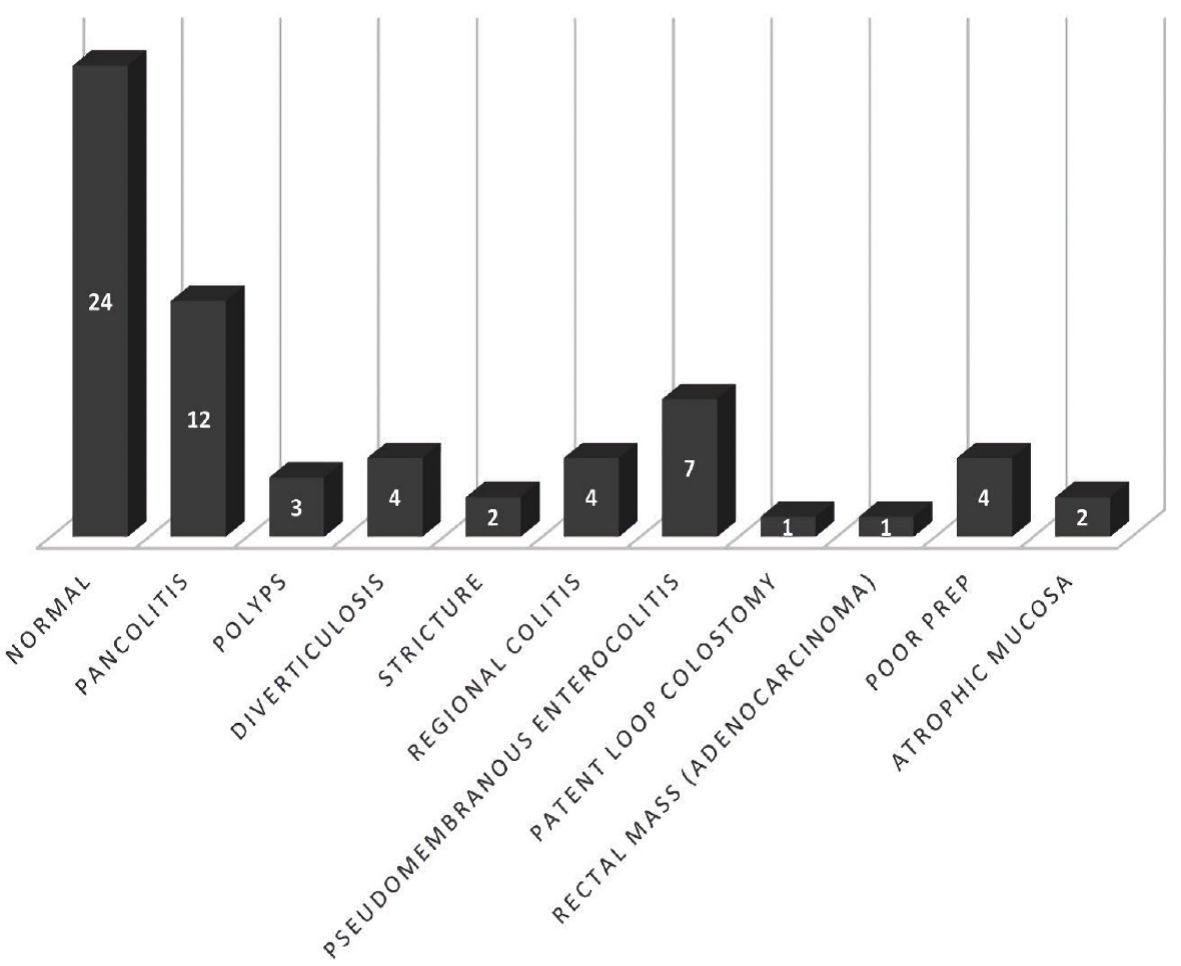
Figure 1. Colonoscopy findings.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 14, Number 4, August 2021, pages 237-243
Fecal Microbiota Transplantation in Patients With Recurrent Clostridium difficile Infection: A Four-Year Single-Center Retrospective Review
Figure

Tables
| N (%) | |
|---|---|
| PPI: proton pump inhibitors; CDI: Clostridium difficile infection; IBD: inflammatory bowel disease; FMT: fecal microbiota transplant. | |
| Gender | |
| Male | 27 (42.2%) |
| Female | 37 (57.8%) |
| Referral | |
| In-house | 55 (85.9%) |
| Outside | 9 (14.1%) |
| Inpatient | 37 (57.8%) |
| Outpatient | 27 (42.2%) |
| Comorbidities and risk factors | |
| History of PPI use | 27 (42.2%) |
| Antibiotics used before CDI | 38 (59.4%) |
| Use of immunosuppressants | 12 (18.8%) |
| Diabetes | 17 (26.6%) |
| Hypertension | 38 (59.4%) |
| Immunocompromised state including IBD | 25 (39.1%) |
| Antibiotics used for the treatment of CDI before FMT | |
| Vancomycin alone | 14 (21.8%) |
| Fidaxomicin alone | 0 (0%) |
| Metronidazole alone | 2 (3.1%) |
| Vancomycin + metronidazole | 16 (25.0%) |
| Vancomycin + fidaxomicin | 10 (15.6%) |
| Fidaxomicin + metronidazole | 0 (0%) |
| No antibiotic therapies | 1 (1.5%) |
| All three | 21(42.1%) |
| Antibiotics stopped > 48 h before FMT | 46 (71.9%) |
| Adverse events from FMT | 12 (18.8%) |
| Abnormal colonoscopy findings | 39 (60.9%) |
| N (%) | |
|---|---|
| CDI: Clostridium difficile infection; FMT: fecal microbiota transplant. | |
| Improvement in 2 months | 48 (75.0%) |
| FMT failure | 10 (15.6%) |
| Patients lost to follow-up | 6 (9.4%) |
| Recurrence of CDI after 2 months | 26 (40.0%) |
| Demographic variables | N | Successa | P value | Recurrence | P value |
|---|---|---|---|---|---|
| aSuccess was defined as more than 50% improvement of symptoms in 2 months. Values were shown as n (%). CDI: Clostridium difficile infection; FMT: fecal microbiota transplant; PPI: proton pump inhibitor. | |||||
| Gender | |||||
| Male | 27 | 23 (85.3%) | 10 (37.0%) | ||
| Female | 37 | 25 (67.6%) | 0.24 | 16 (43.2%) | 0.61 |
| Referral | |||||
| In-house | 55 | 43 (78.2%) | 21 (38.2%) | ||
| Outside | 9 | 5 (55.6%) | 0.002 | 5 (55.6%) | 0.32 |
| Inpatient | 37 | 30 (81.1%) | 17 (45.9%) | ||
| Outpatient | 27 | 18 (66.7%) | 0.09 | 9 (33.3%) | 0.31 |
| Comorbidities and risk factors | |||||
| History of PPI use | 27 | 21 (77.8%) | 0.66 | 14 (51.9%) | 0.11 |
| Antibiotics used before CDI | 38 | 27 (71.1%) | 0.05 | 14 (36.8%) | 0.45 |
| Use of immunosuppressants | 12 | 10 (83.3%) | 0.72 | 8 (66.7%) | 0.04 |
| Diabetes | 17 | 12 (70.6%) | 0.87 | 6 (35.3%) | 0.62 |
| Hypertension | 38 | 30 (78.9%) | 0.68 | 18 (47.4%) | 0.18 |
| Immunocompromised state | 25 | 19 (76%) | 0.72 | 11 (44.0%) | 0.66 |
| Antibiotics stopped > 48 h before FMT | 46 | 34 (73.9%) | 0.90 | 17 (37.0%) | 0.19 |
| Adverse events from FMT | 12 | 7 (58.3%) | 0.17 | 3 (25.0%) | 0.22 |
| Abnormal colonoscopy findings | 37 | 26 (70.3%) | 0.53 | 15 (40.5%) | 0.62 |
| N (%) | |
|---|---|
| CDI: Clostridium difficile infection; FMT: fecal microbiota transplant. | |
| Antibiotic use before CDI recurrence | 9 (34.6%) |
| Immunosuppressant use before recurrence | 8 (30.7%) |
| Crohn’s disease | 3 (11.5%) |
| Ulcerative colitis | 3 (11.5%) |
| Organ transplant recipients | 1 (3%) |
| Treatment with repeat FMT | 18 (69.2%) |
| Treatment with antibiotics | 7 (26.9%) |