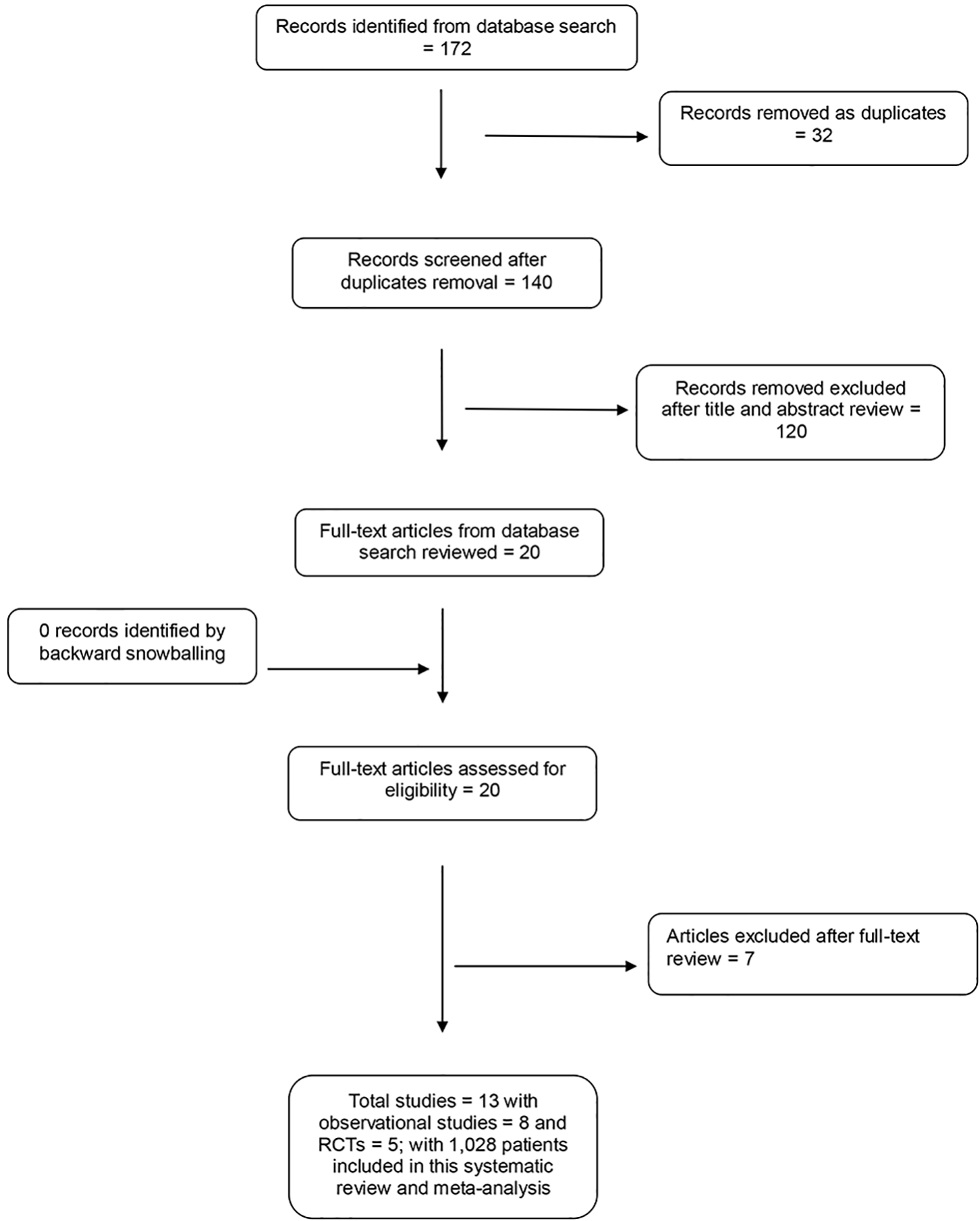
Figure 1. PRISMA flow diagram.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 13, Number 6, December 2020, pages 260-268
Safety and Efficacy of Nitazoxanide-Based Regimen for the Eradication of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis
Figures

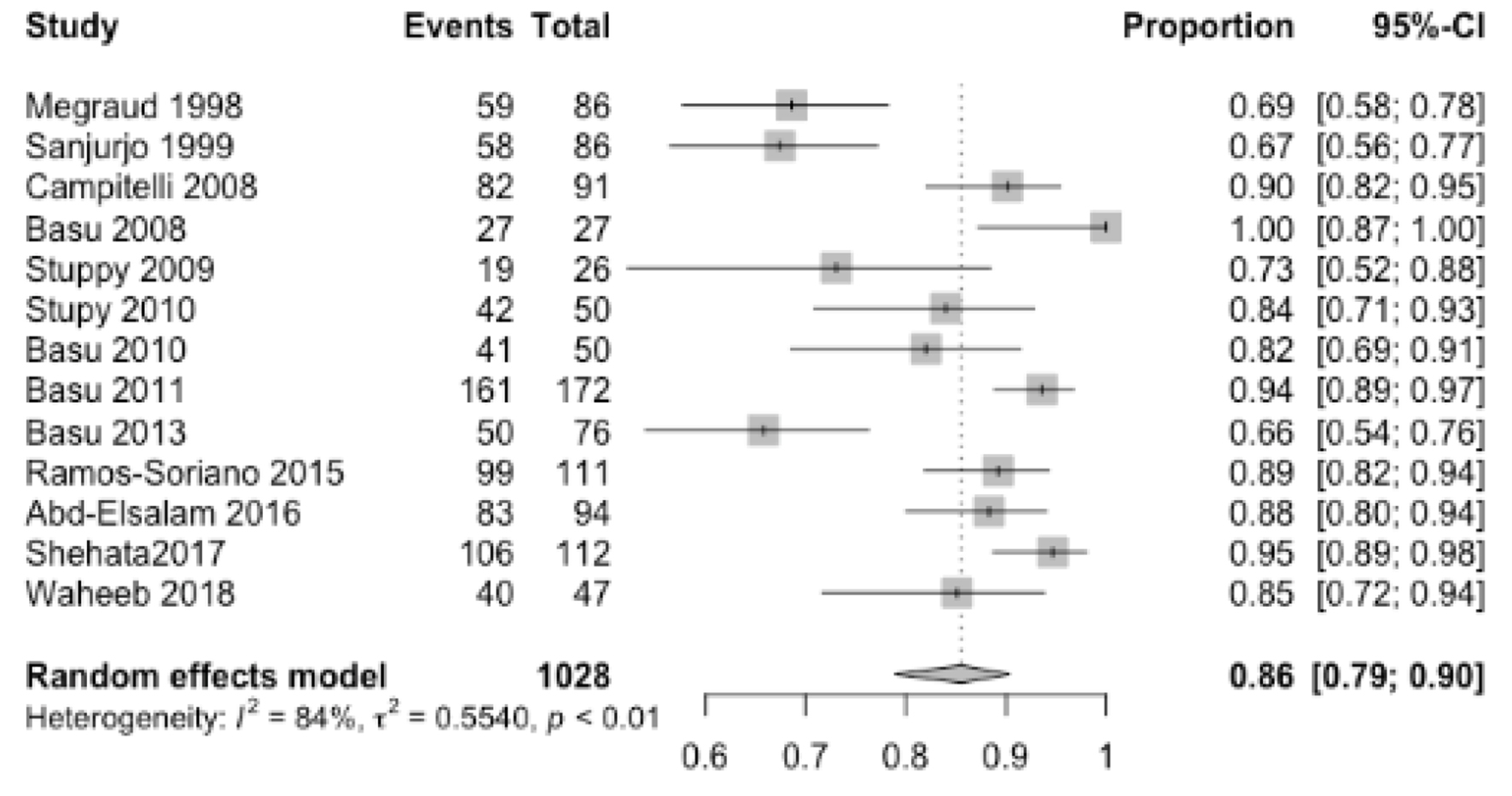
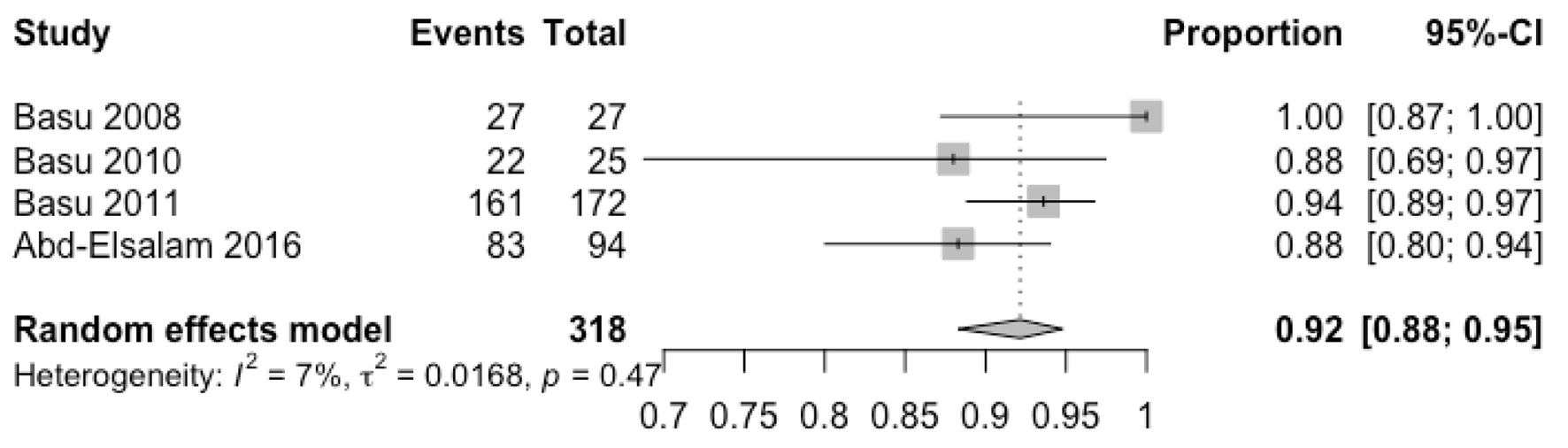
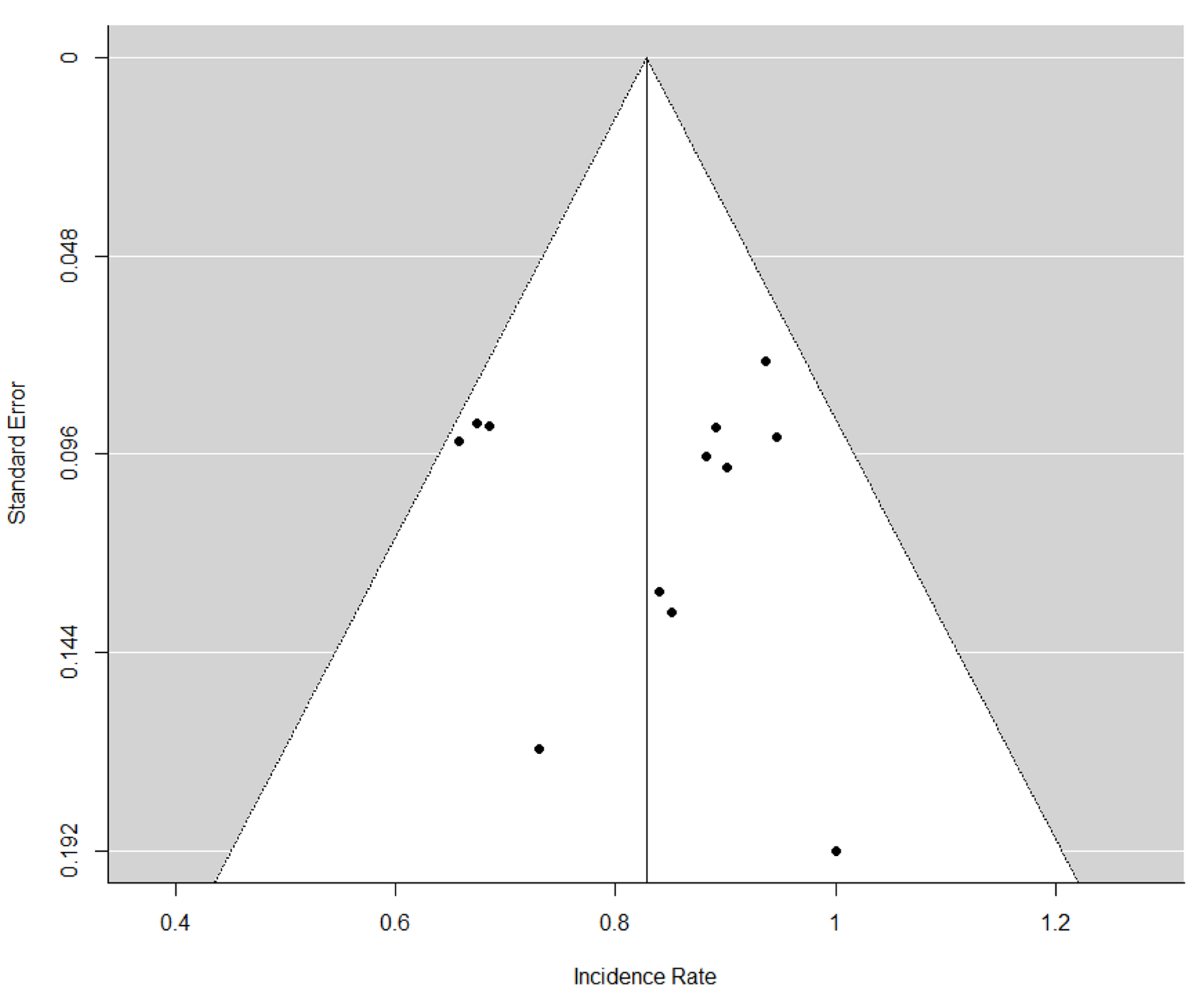
Table
| Authors, year of publication and country | Study design and sample size | Patient population/prior treatments | Eradication regimen | Dose of NTZ and duration | ER and criteria for eradication | Adverse events |
|---|---|---|---|---|---|---|
| HP: Helicobacter pylori; BID: twice a day; TID: three times a day; BS: bismuth subcitrate; LP: lansoprazole; PPI: proton pump inhibitor; GI: gastrointestinal; NTZ: nitazoxanide; RCT: randomized controlled trial; ER: eradication rate. | ||||||
| Megraud et al, 1998, Egypt [16] | Open phase II dose ranging pilot study | Previously untreated dyspeptic patients diagnosed with HP | NTZ + omeprazole 20 mg/day | 500 mg BID for 14 days; 500 mg TID for 7 days; or 1,000 mg BID for 7 days. | ER was 65% in 500 BID for 14 days group, 58% in 500 mg TID for 7 days group and 83% in 1 g BID group. Patients were included if HP was positive in two of the following: histology, culture and serology. Same tests were repeated (except for serology) 4 weeks after treatment to confirm eradication. Urea breath test was also performed after treatment. | Minor adverse events were reported in 15 patients. |
| Sanjurjo et al, 1999, Mexico [25] | Open prospective, longitudinal study | Previously untreated | NTZ + BS + LP 30 mg BID | 500 - 1,000 mg in three different regimens | ER was 41.37% in NTZ 1 g + BS 240 mg + PPI for 7 days group, 75% in NTZ 500 + BS 120 mg + PPI for 14 days group and 83% in NTZ 1,000 + BS 240 mg and PPI for 14 days group. Eradication was confirmed with negative urea breath test 6 weeks after treatment. | No serious adverse events were reported. |
| Campitelli et al, 2008, Argentina [22] | Open label study | Previously untreated | NTZ + LP + amoxicillin for 7 days | 500 mg BID | ER was 90%. Eradication was confirmed with negative urea breath test 6 - 8 weeks after treatment. | No major adverse events were reported. |
| Basu et al, 2008, USA [23] | Open label prospective study | Failed prior treatment regimen | Levofloxacin, esomeprazole, NTZ and doxycycline for 10 days | 500 mg BID | ER was 100% in per protocol analysis (90% in intention to treat analysis). Eradication was confirmed with negative stool antigen test in 2 weeks after treatment. | Nausea and bloating were reported in two patients. Non-specific itching was reported in one patient. |
| Stuppy, 2009, USA [18] | RCS | Previously untreated | Sucralfate + NTZ for 14 days | 1,000 mg BID | ER was 73%. Eradication was confirmed with negative salivary HP antibodies 1 to 6 months after the end of therapy. | Yellowing of urine and GI discomfort were reported in few patients |
| Stuppy, 2010, USA [19] | RCS | 39 previously untreated; 11 failed prior treatment | Sucralfate + NTZ for 14 days | 1,000 mg BID | ER was 84%. Eradication was confirmed with negative salivary HP antibodies 1 - 6 months after the end of therapy. | Yellowing of urine and GI discomfort were reported in few patients. |
| Basu et al, 2011, USA [14] | Randomized open label trial | Previously untreated | Levofloxacin, omeprazole, NTZ and doxycycline for 7 days or 10 days | 500 mg BID | ER was 93.6% in per protocol analysis (89.4% in intention to treat analysis). Eradication was confirmed with negative stool antigen test in 4 weeks after treatment. | Minor adverse events (headache, abdominal pain, nausea, diarrhea, etc.) |
| Basu et al, 2013, USA [21] | Randomized open label clinical pilot study | Failed prior treatment regimens | Rifabutin + NTZ + omeprazole + doxycycline (ROAD) or macrodantin + NTZ + doxycycline + omeprazole (MOAD) | 500 mg BID | ER was 65.7% overall (72% in ROAD group and 52% in MOAD group). | Minor adverse events like nausea, vomiting, diarrhea and headache. |
| Ramos-Soriano and Black, 2015, USA [15] | RCS | Previously untreated pediatric population | NTZ for 3 days + azithromycin and cefixime or any third-generation cephalosporin for 7 - 10 days + PPI for 30 days | Mean dose of 327.5 mg BID | ER was 89.2%. Eradication was confirmed with negative urea breath test with resolution of symptoms within 4 months of treatment. | 10% patients had minor adverse events like nausea, vomiting and abdominal cramping. |
| Abd-Elsalam et al, 2016, Egypt [13] | Open label study | Failed clarithromycin-based regimen in the past | Levofloxacin, omeprazole, NTZ and doxycycline for 14 days | 500 mg BID | 88.3% in per protocol analysis (83% in intention to treat analysis). Eradication was confirmed with negative stool antigen test in 6 weeks after treatment. | Minor adverse events (headache, abdominal pain, nausea, diarrhea, etc.) were noted in 21 patients. |
| Shehata et al, 2017, Egypt [17] | RCT | Previously untreated patients | Clarithromycin + NTZ and omeprazole for 14 days | 500 mg BID | ER was 94.6%. Eradication was confirmed with negative stool antigen test in 6 weeks after treatment. | Abdominal pain, nausea, constipation and dizziness |
| Waheeb et al, 2018, Egypt [24] | RCT | 25 previously untreated patients and 22 patients failed treatment in the past. | Clarithromycin + NTZ + amoxicillin and omeprazole for 14 days | 500 mg BID | 85% (92% in previously untreated and 77.3% in patients who failed prior treatment). Eradication was confirmed with negative stool antigen test in 6 weeks after treatment. | Minor adverse events like nausea and vomiting were reported in few patients. |
| Basu et al, 2010, USA [20] | RCT | Previously untreated patients | Levofloxacin, omeprazole, NTZ and doxycycline for 7 days or dexlansoprazole + moxifloxacin + amoxicillin + doxycycline + NTZ for 4 days | 500 mg BID | 82% eradication in intention to treat analysis. Eradication was confirmed with negative stool antigen test 30 days after treatment. | Minor rash, dizziness, diarrhea and palpitations |