Figures
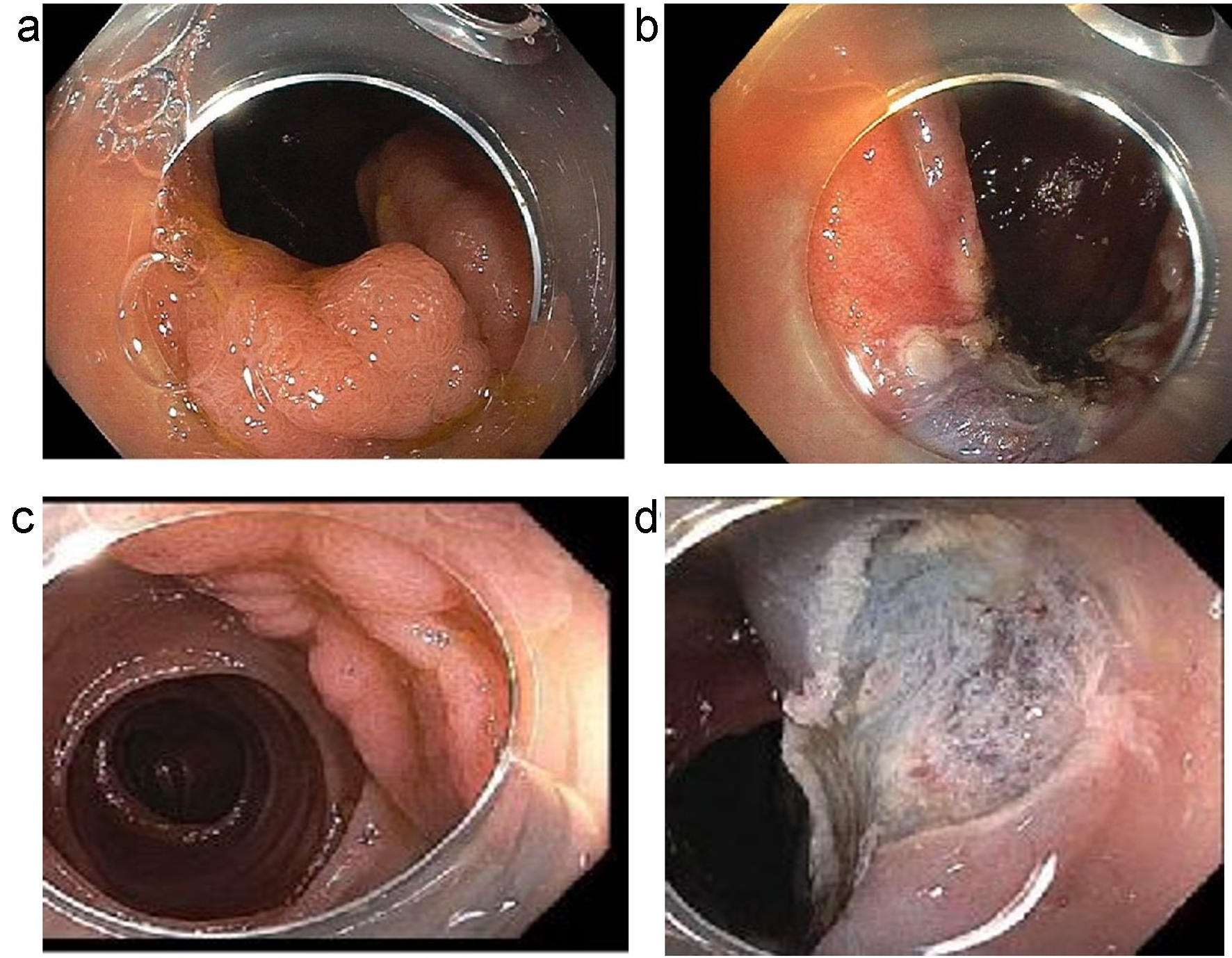
Figure 1. (a, b) An example of a laterally spreading Paris 0-IIa lesion with slight elevation. (a) Before polypectomy. (b) Post-polypectomy. (c, d) An example of a sessile slightly elevated lesion (Paris IIa-Is). (c) Before polypectomy. (d) Post-polypectomy.
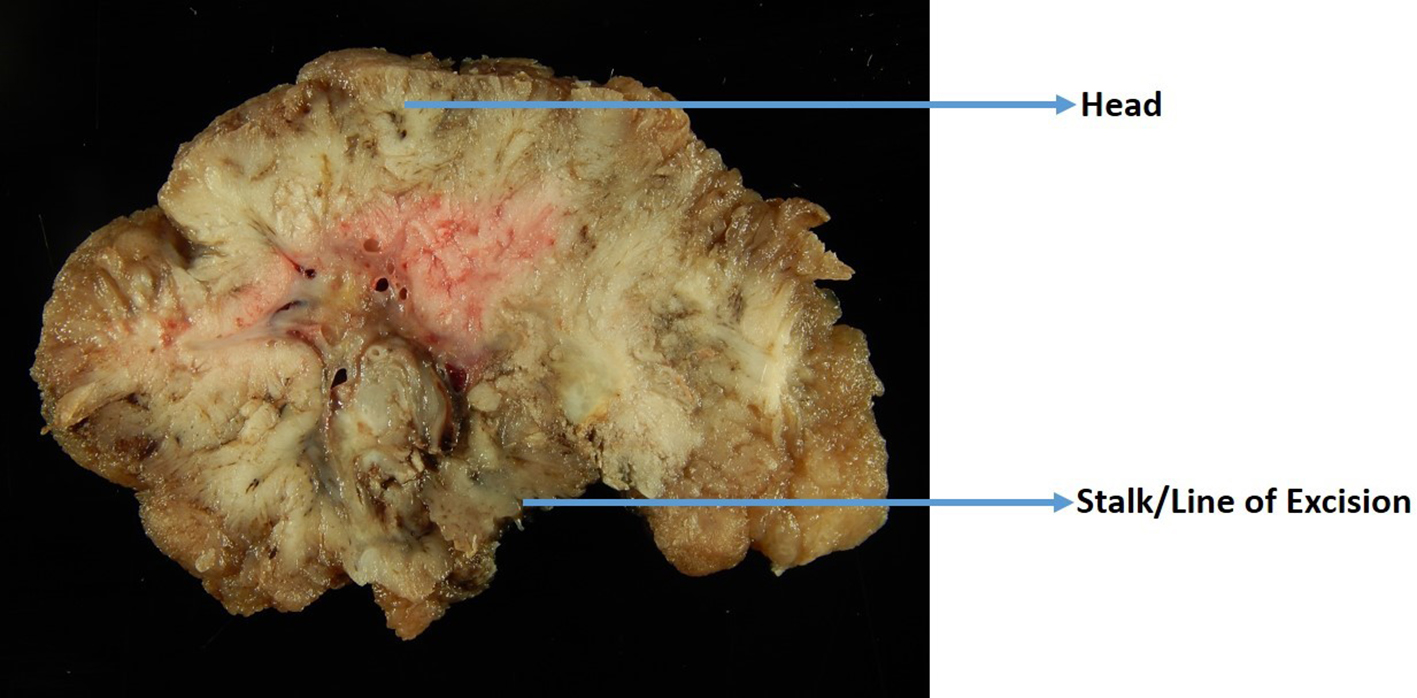
Figure 2. A gross photo of a large polyp that is cut perpendicular to the line of excision.
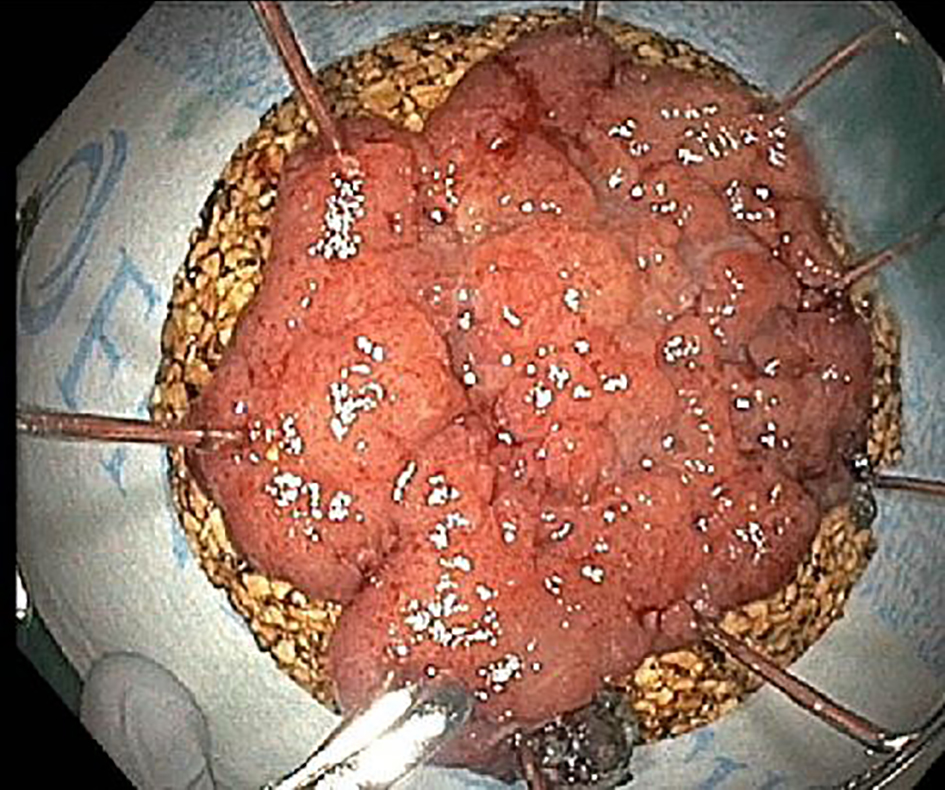
Figure 3. Endoscopic dissection specimens are usually pinned out flat on a cork or foam board by the endoscopist. All ESD specimens have peripheral and deep/vertical resection margins. ESD: endoscopic submucosal dissection.
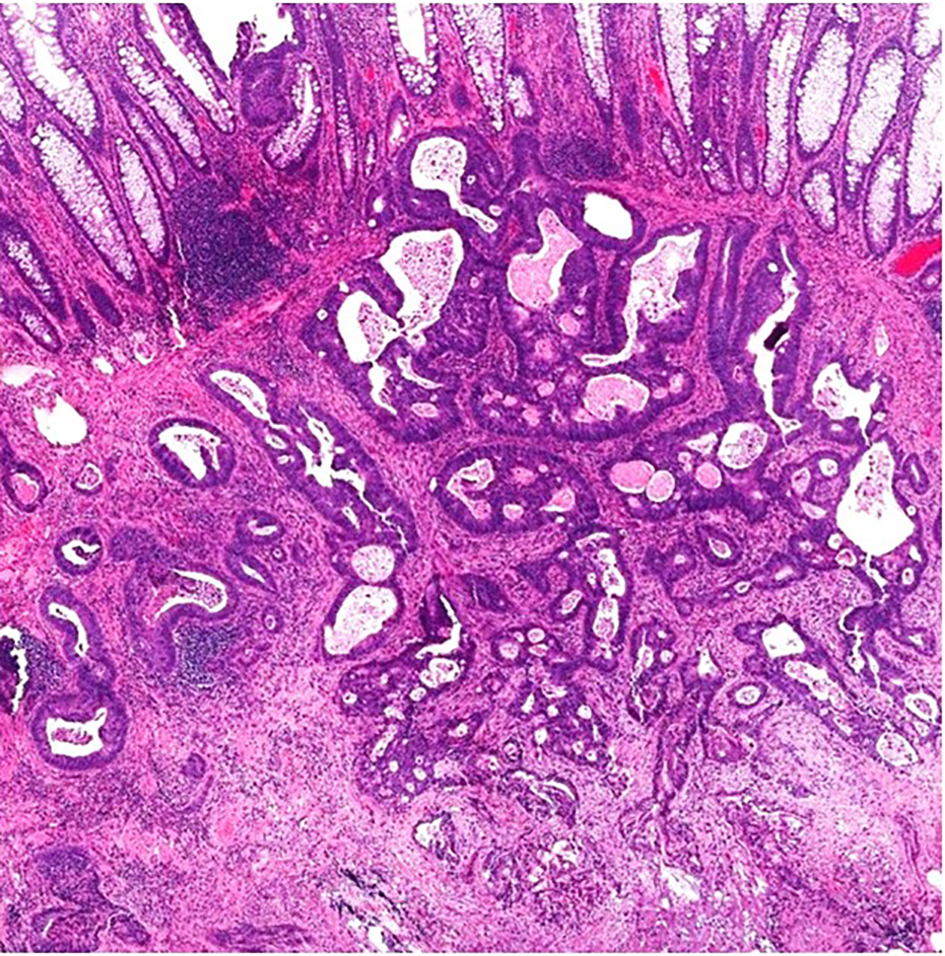
Figure 4. A malignant polyp in a well-oriented specimen showing irregular, ill-formed glands with luminal necrosis below the muscularis mucosae. There is prominent desmoplasia in the deep aspect of the lesion.
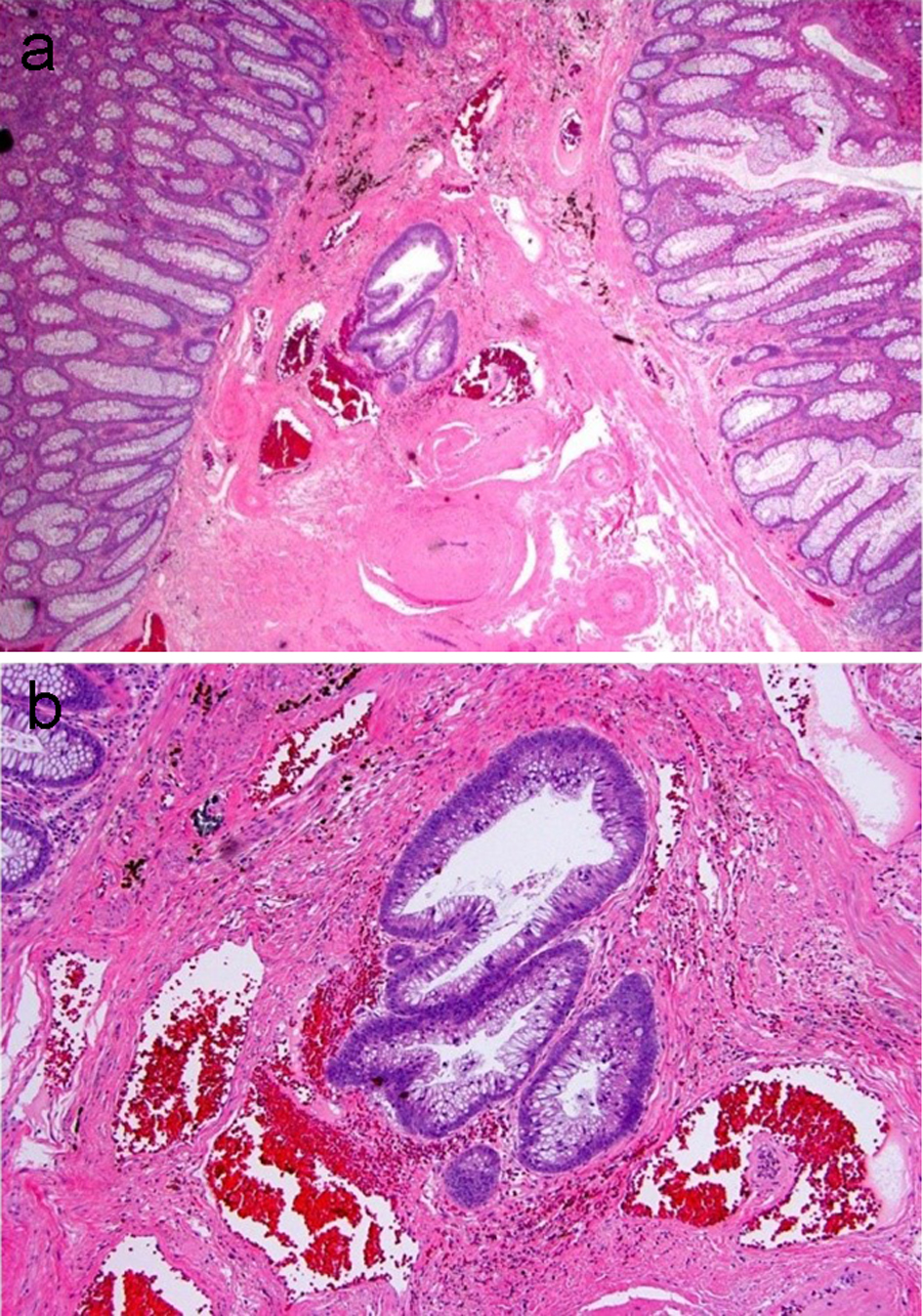
Figure 5. An adenomatous polyp with misplaced adenomatous epithelium within the submucosa. (a) Well-formed adenomatous glands are present below the muscularis mucosa. (b) Higher magnification reveals glands with low-grade dysplasia and a rounded contour surrounded by lamina propria. Hemosiderin is present in the adjacent stroma.
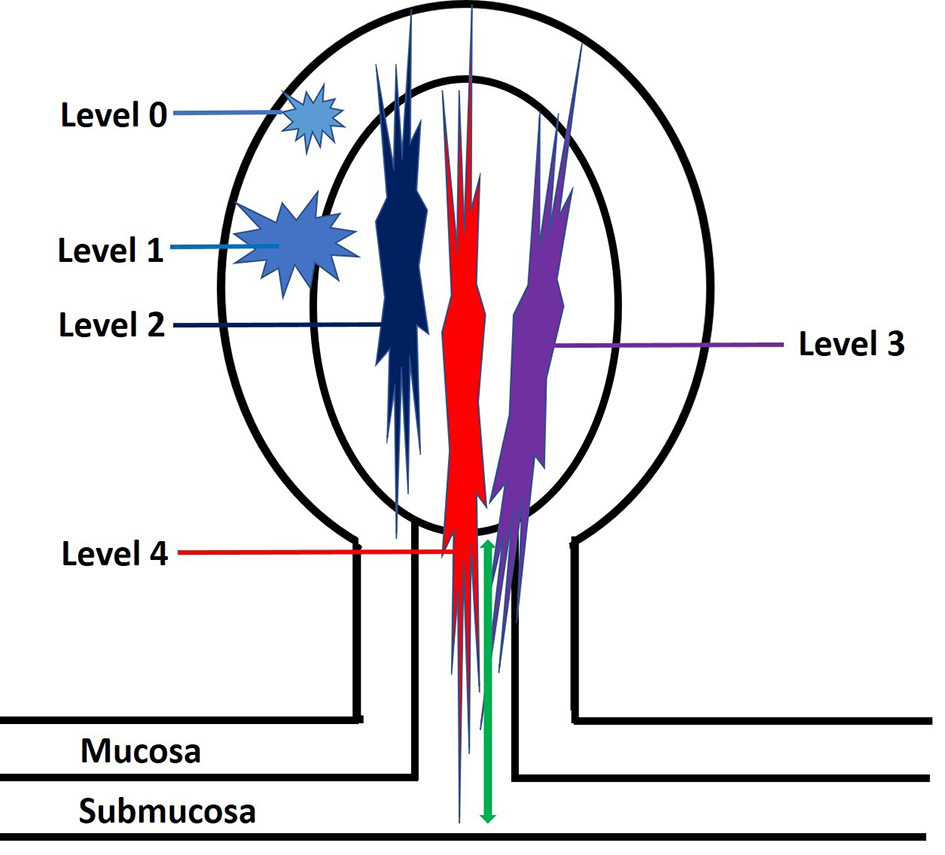
Figure 6. An illustration of Haggitt levels for pedunculated polyps. Level 0: tumor is limited to the mucosa without invasion into the submucosa. Level 1: tumor invades into the submucosa but is limited to the head of the polyp. Level 2: tumor invades into the polyp’s neck (the junction of the head and the stalk). Level 3: tumor invades into the stalk. Level 4: tumor invades beyond the stalk of the polyp but remains above the muscularis propria. The depth of submucosal invasion is measured from the polyp’s neck to the deepest portion of the invasion (green arrow).
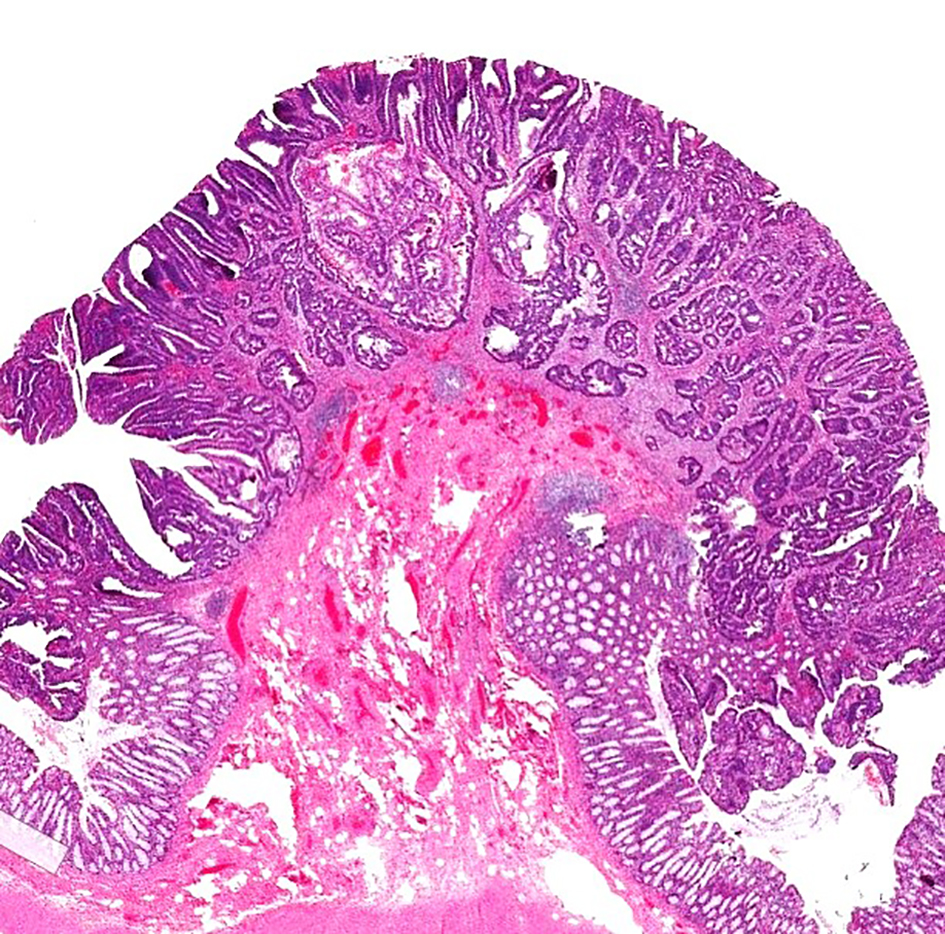
Figure 7. H&E stain of a pedunculated malignant polyp. The tumor invades into the submucosa but is confined to the head of the polyp (Haggitt level 1). H&E: hematoxylin and eosin.
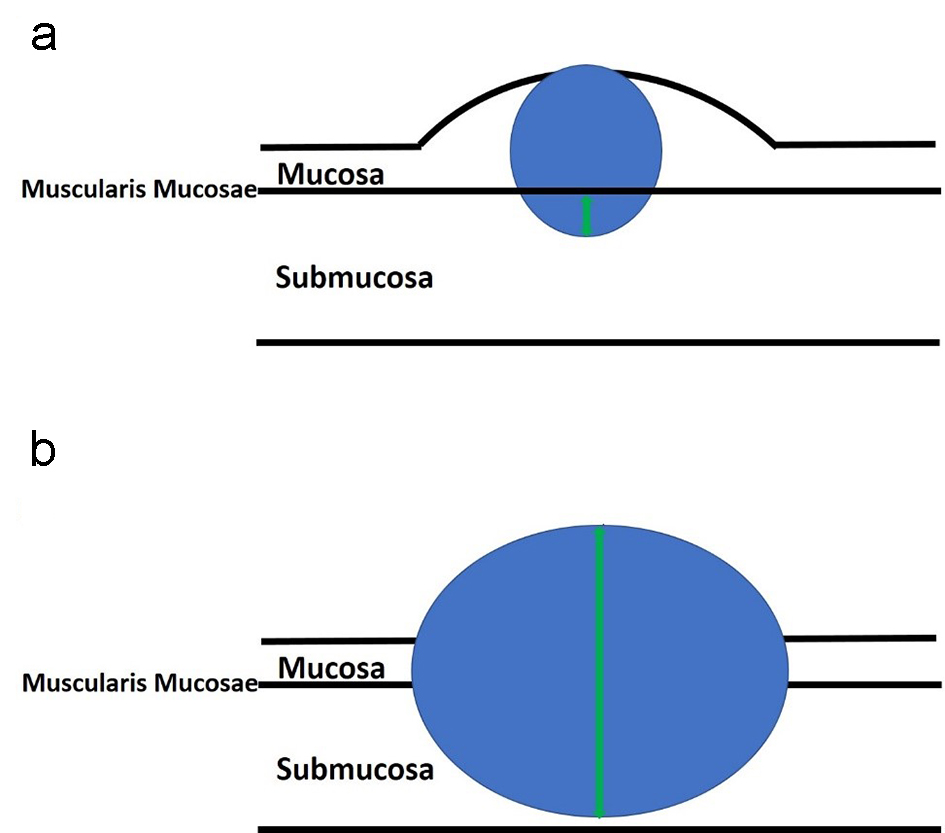
Figure 8. An illustration of sessile polyps and measurements for the depth of submucosal invasion. Sessile polyps grow in a flattened conformation over the mucosa. (a) The depth of submucosal invasion is usually measured from the muscularis mucosae to the deepest aspect of invasion (green arrow). (b) However, if the muscularis mucosae layer is completely obliterated by the tumor, the measurement is taken from the most superficial portion of invasion down to its deepest extent of invasion (green arrow).
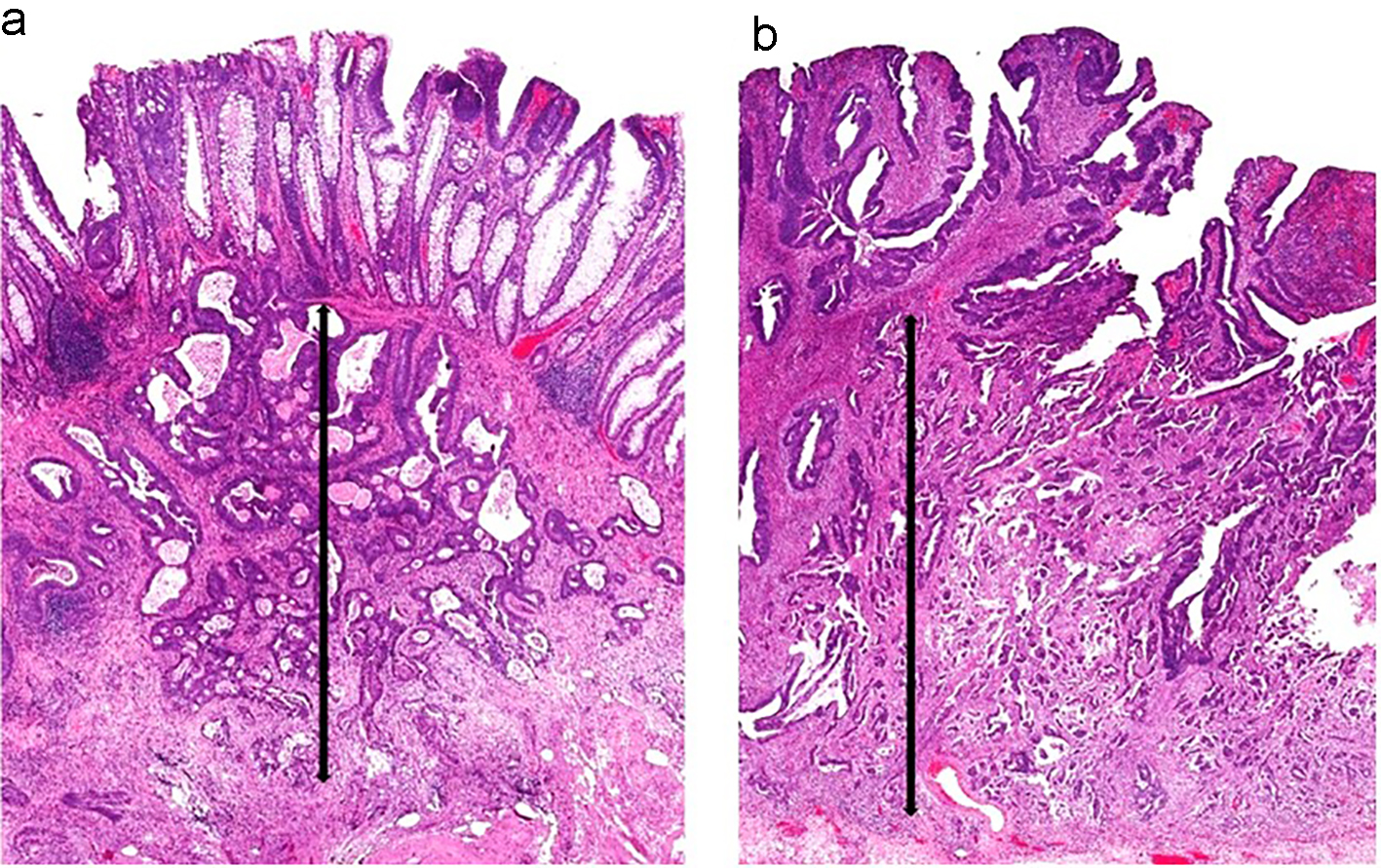
Figure 9. Two sessile polyps and their respective measurements for depth of submucosal invasion. (a) The muscularis mucosae are apparent in this polyp. The depth of submucosal invasion is measured from the muscularis mucosae to the deepest invasive component (black arrow). (b) The muscularis mucosae are obliterated by carcinoma in this polyp. Thus, the submucosal invasion measurement is taken from the most superficial aspect of tumor invasion down to its deepest extension (black arrow).
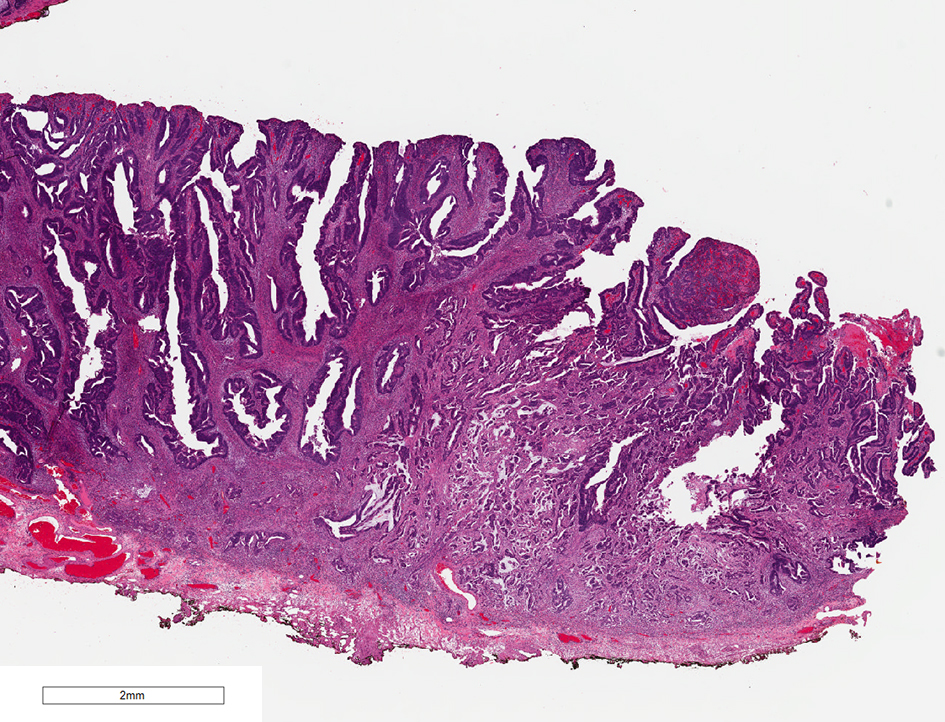
Figure 10. H&E stain of a malignant polyp. Grade 3 adenocarcinoma is a poorly differentiated carcinoma which contains less than 50% glandular differentiation. H&E: hematoxylin and eosin.
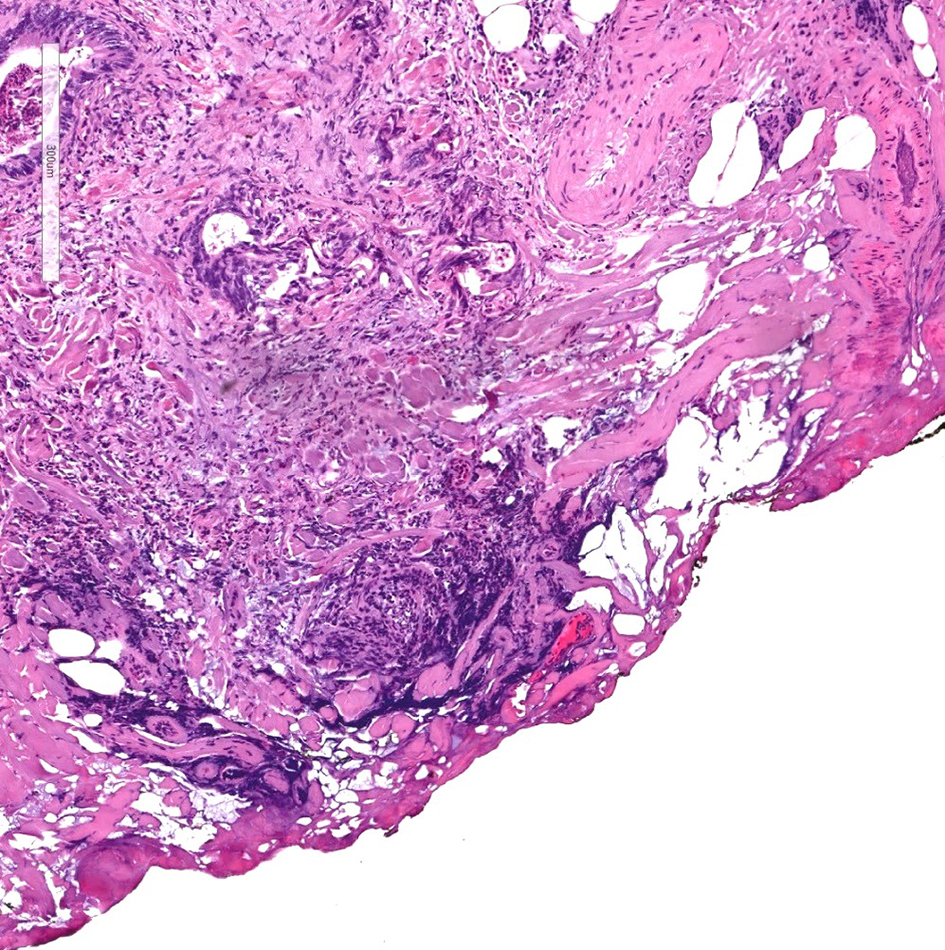
Figure 11. H&E stain of a cauterized polyp margin with tumor. A positive deep margin in a malignant polyp is defined as the presence of invasive adenocarcinoma within 1 mm of the inked or cauterized deep margin. H&E: hematoxylin and eosin.
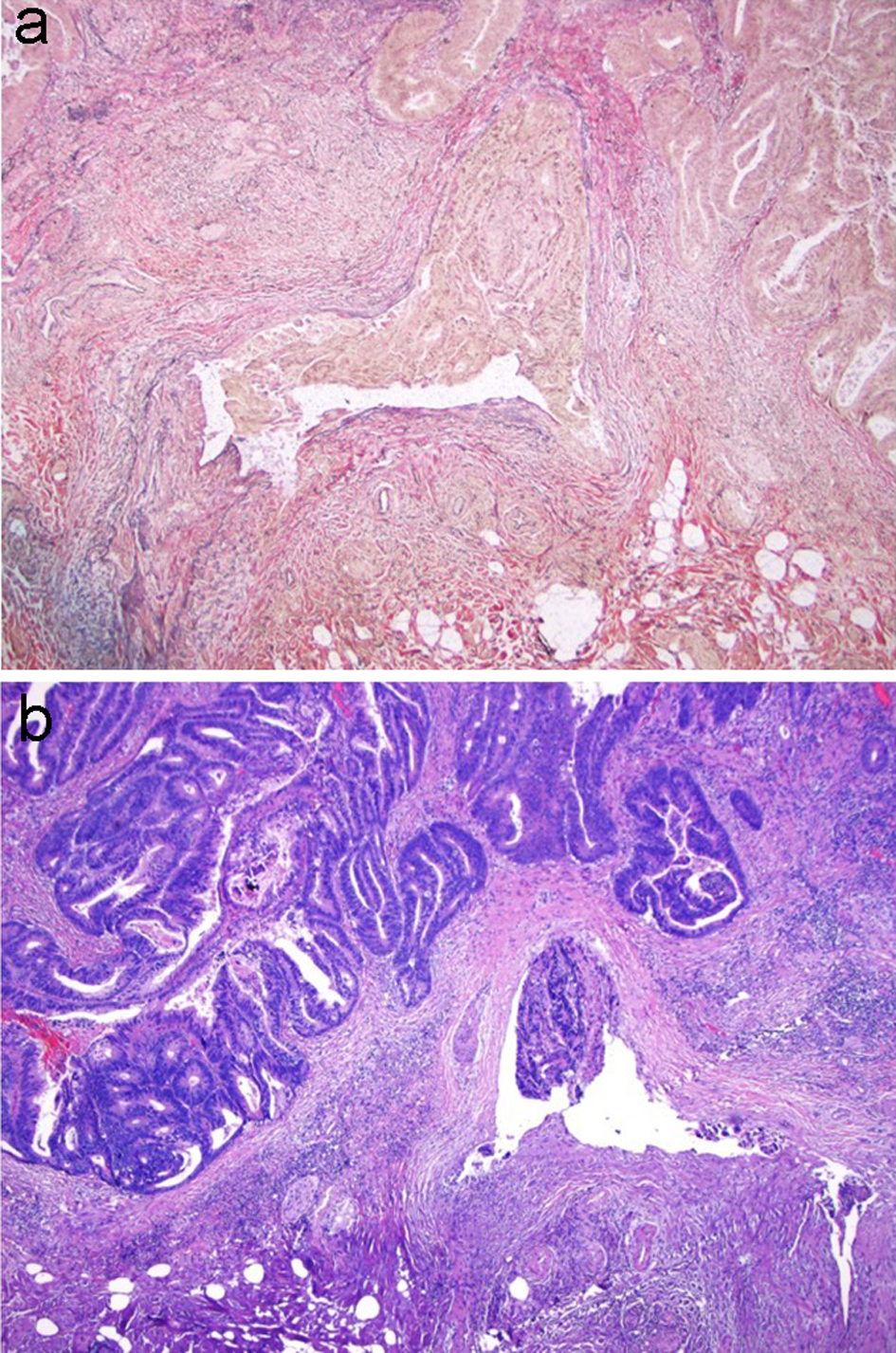
Figure 12. VVG stain is used to evaluate for large vessel invasion. (a) VVG stain reveals venous invasion in this case of invasive adenocarcinoma. (b) H&E stain demonstrates orphan arterioles associated with adjacent venous invasion. VVG: Verhoeff-Van Gieson; H&E: hematoxylin and eosin.
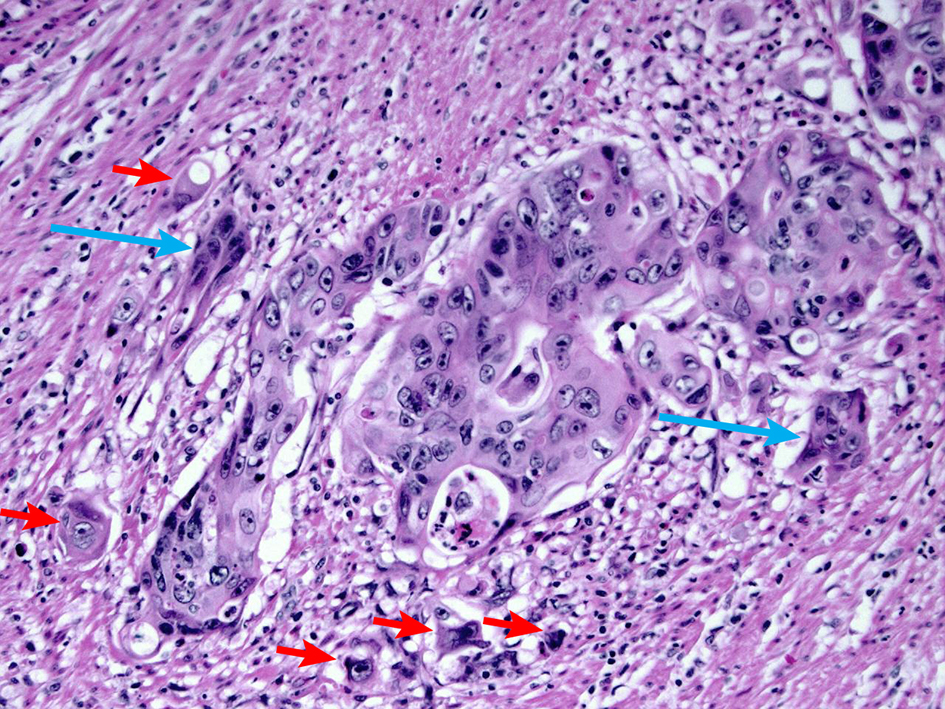
Figure 13. H&E stain demonstrating tumor budding. Tumor budding is defined as the presence of single tumor cells or small clusters of less than five tumor cells at the advancing front of the tumor (short arrows). Cancer clusters of more than five tumor cells showing invasion into the stroma without gland formation are defined as poorly differentiated clusters (long arrow). A high level of tumor budding is associated with nodal metastasis in early colorectal adenocarcinoma. A high level of poorly differentiated clusters is associated with a high recurrence rate. H&E: hematoxylin and eosin.
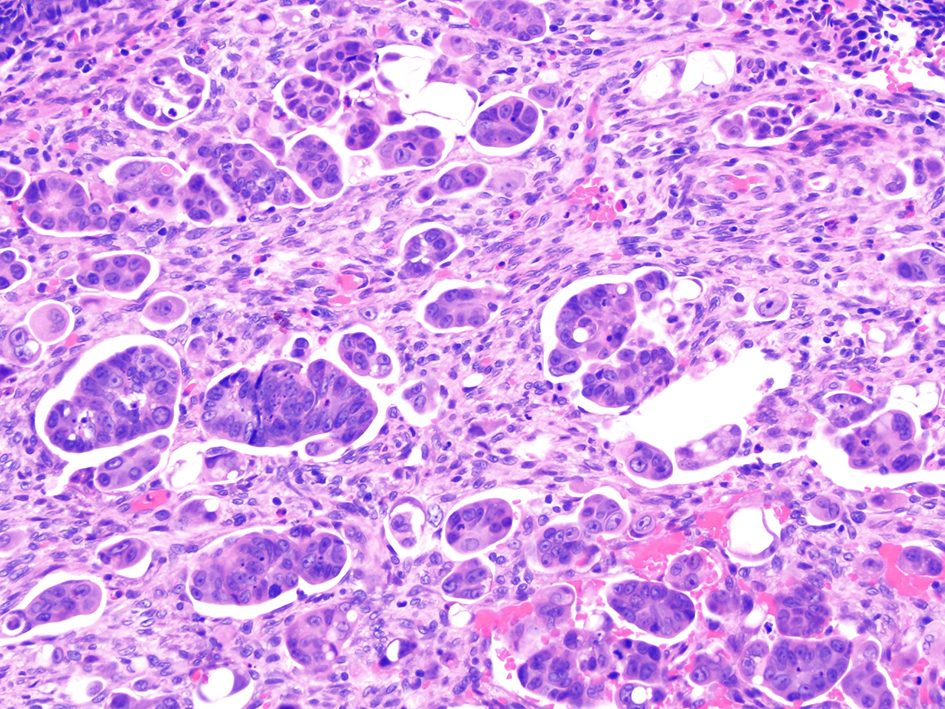
Figure 14. Invasive colorectal adenocarcinoma demonstrating a micropapillary growth pattern. A micropapillary pattern is characterized by small and tight clusters of tumor cells with surrounding cleft-like spaces. This histologic feature is associated with lymphovascular invasion and nodal metastasis.
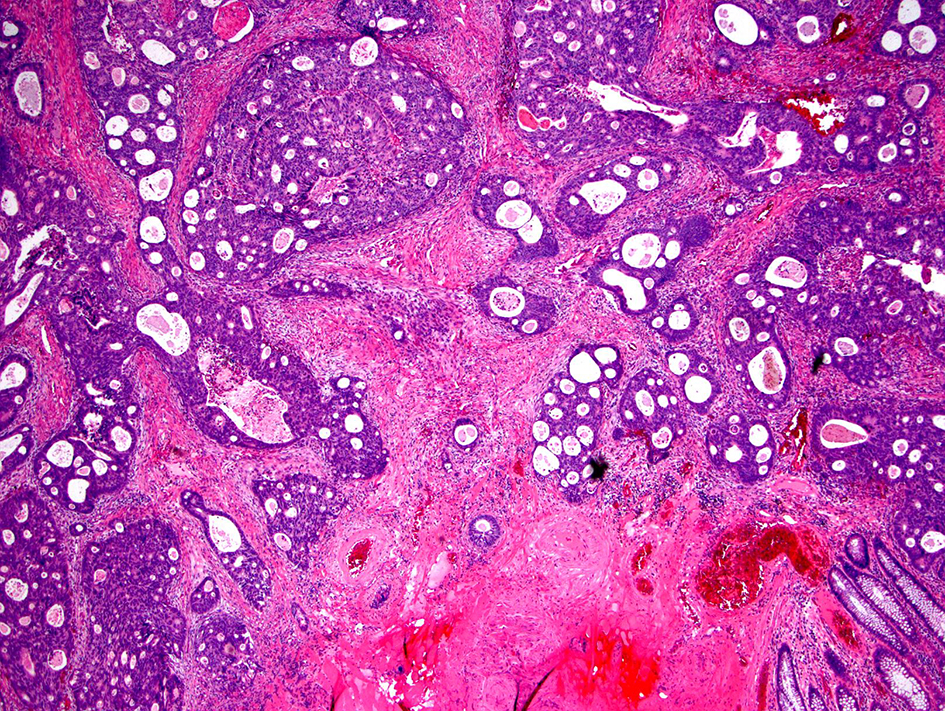
Figure 15. Invasive colorectal adenocarcinoma with an intermediate desmoplastic stromal reaction. Intermediate desmoplasia is characterized by the presence keloid-like collagen and myxoid stroma less than a microscopic field of a × 40 objective lens.
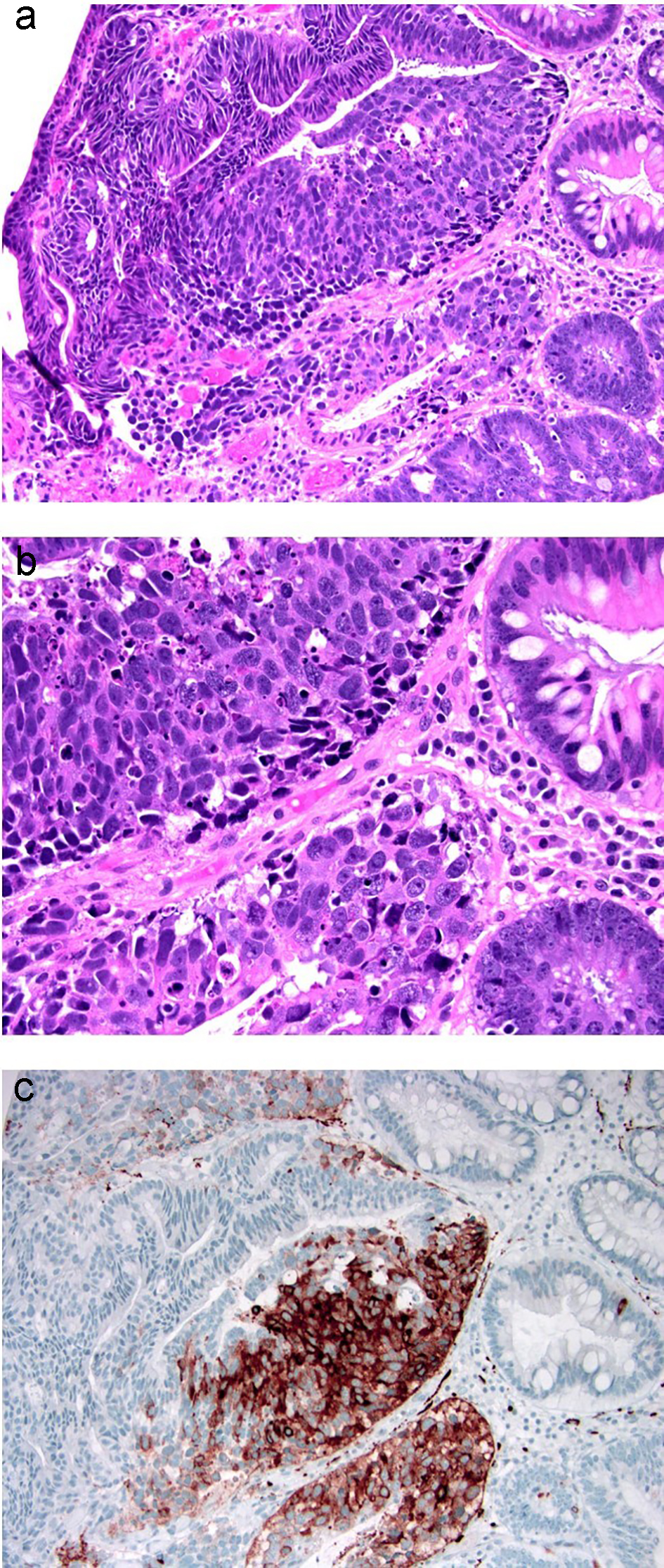
Figure 16. Colonic adenoma with focal intramucosal high-grade neuroendocrine carcinoma. (a, b) Tubular adenoma with a single cluster of cells exhibiting extremely high nuclear to cytoplasmic ratio and brisk apoptosis, breaching the basement membrane of the glands. (c) This focus is strongly positive for synaptophysin. Ki-67 immunolabeling showed a high proliferative index of up to 90% (not depicted).
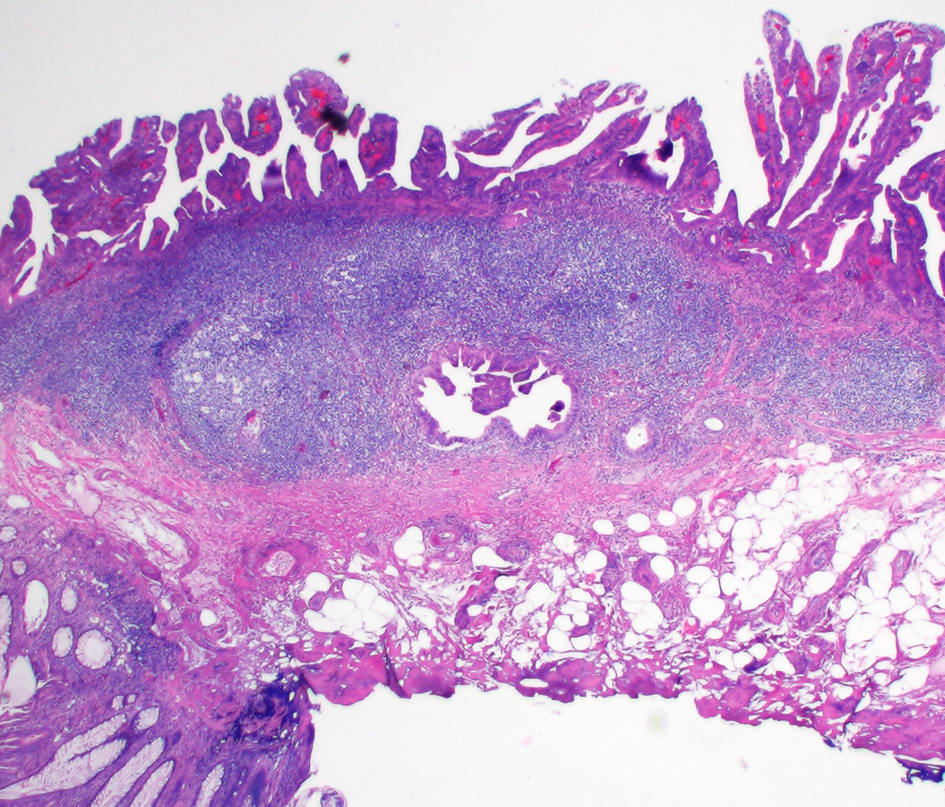
Figure 17. Colonic glandular neoplasia can involve submucosal lymphoglandular complexes (LGCs) and pose diagnostic challenges. In this case, the focus of submucosally located dysplastic glands is entirely surrounded by lymphoid aggregates. This focus is also in continuity with the overlying surface adenoma on additional levels (not depicted).
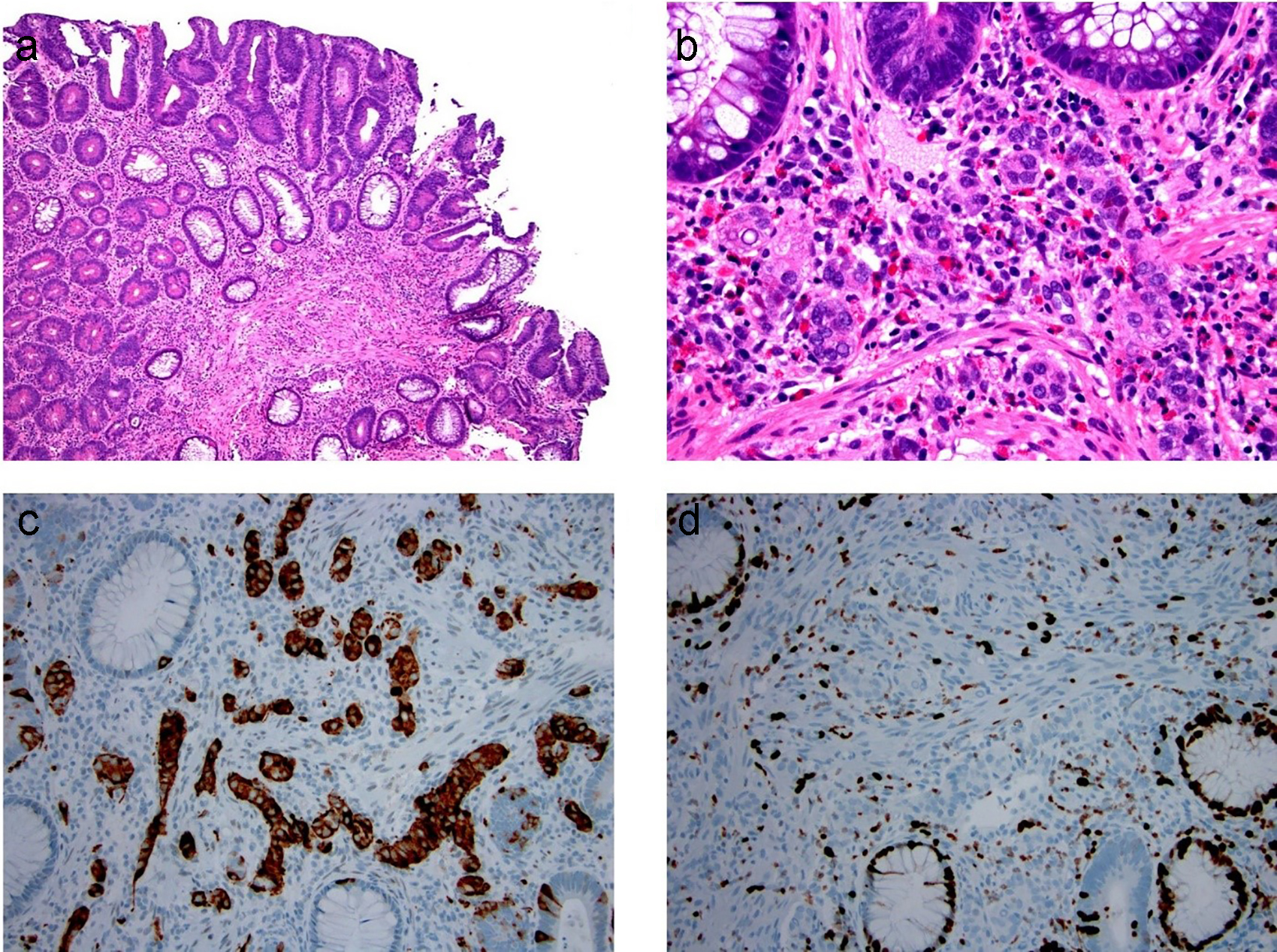
Figure 18. Composite intestinal adenoma-microcarcinoid consists of colonic adenoma and microcarcinoid. (a, b) Microcarcinoids in these lesions are more commonly well-differentiated neuroendocrine cells arranged in small clusters, glands, or cords in the basal lamina propria. (c, d) These cells are positive for synaptophysin (c) and have low Ki-67 immunolabeling (d).
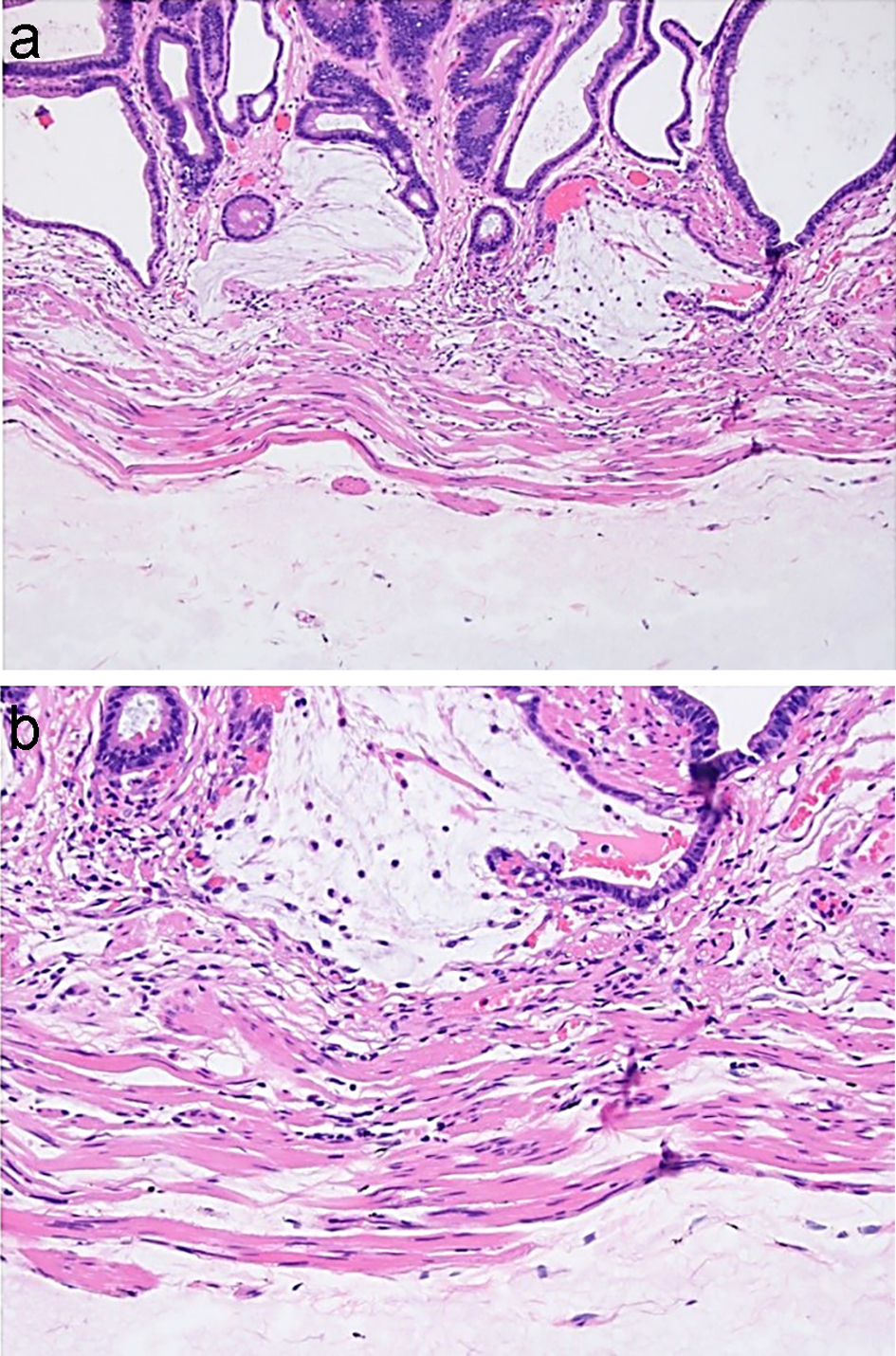
Figure 19. Orise in the polypectomy specimen removed immediately after Orise injection may closely mimic mucin. (a, b) Mucin associated with crypt rupture is seen above the muscularis mucosae. Orise gel can be seen below the muscularis mucosae.
Tables
Table 1. The Paris Classification of Colorectal Neoplastic Lesions
| Endoscopic appearance | Description | Paris classification |
|---|
| Protruded lesion | Sessile polyps | Is |
| Pedunculated polyps | Ip |
| Subpedunculated polyps | Isp |
| Flat lesion | Flat elevation | IIa |
| Completely flat | IIb |
| Slight depression | IIc |
| Flat elevation with central depression | IIa + IIc |
| Central depression and raised edge | IIc + IIa |
| Excavated lesion | Excavated/ulcerated | III |
Table 2. Histologic Features Distinguishing Misplacement of Dysplastic Epithelium and Submucosal Invasion
| Features | Misplacement of dysplastic epithelium | Submucosal invasion |
|---|
| Pedunculation | Usually present | May be absent |
| Architecture at low power | Lobular, round, or smooth contour | Irregular shape, haphazard distribution |
| Lamina propria around crypts | May be present | Absent |
| Connection of deep glands with adenoma | Can be demonstrated, but may need deeper levels | May be absent |
| Arrangement of submucosal glands | Regular | Haphazard |
| Shape of submucosal glands | Round and regular | Irregular with incomplete lumen “abortive” glands |
| Cytology of submucosal glands | Similar to the superficial aspect of the lesion | May be worse than superficial aspect of the lesion |
| Hemosiderin | May be present | Absent |
| Desmoplasia | Absent | Present |
| Small nests or single cells | Absent | May be present |
| Mucin pools | Round, smooth, and may be lined by dysplastic epithelium at periphery | Irregular contours, and floating cells may be present |
Table 3. Essential Elements in the Surgical Pathology Report for a Malignant Polyp
| Features | Categories | Methods/criteria | Required/preferably | Clinical significance |
|---|
| EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; MM: muscularis mucosae; LVI: lymphovascular invasion; MMR: mismatch repair; LS: Lynch syndrome. |
| Polyp configuration | Pedunculated | Well-developed stalk | Yes | Recurrence risk |
| Semipedunculated | Short stalk | | |
| Sessile | No stalk | | |
| Procedure | Polypectomy | Acquire information from the endoscopic report | Yes | Recurrence risk |
| EMR | | | |
| ESD | | | |
| Specimen intactness | En bloc | One piece | Yes | Recurrence risk |
| Piecemeal | More than one piece | | |
| Invasive adenocarcinoma | Present | Infiltrative glands below MM | Yes | Nodal metastasis risk; may require resection |
| Absent | All neoplastic glands are above MM | | |
| Size of invasive adenocarcinoma | Continuous variable | Only measure the size of the invasive component | Yes | None |
| Grade | Well | > 95% glandular formation | Yes | Poor differentiation prompts a resection. |
| Moderate | 50-95% glandular formation | | |
| Poor | < 50% glandular formation | | |
| LVI | Small vessel LVI | Tumor cells within endothelium lined spaces | Yes | Presence of LVI prompts a resection. |
| Muscular venous invasion | Tumor cells present in a muscular vein | | |
| Deep margin status | Positive | Invasive carcinoma at or within 1 mm from the margin | Yes | Positive margin prompts a resection. |
| Negative | Invasive carcinoma greater than 1 mm from the margin | | |
| Indeterminate | Applies to piecemeal specimen | | |
| Depth of submucosal invasion | Pedunculated polyp | From the head-stalk junction to the deepest invasion | Yes | Invasion ≥ 1,000 µm into the submucosa prompts a resection. |
| Sessile polyp | From MM to deepest invasion | | |
| | From the most superficial portion of invasion to the deepest invasion if MM absent | | |
| Tumor budding (in an area measuring 0.785 mm2) | Low level | 1 - 4 tumor buds | Yes | Intermediate or higher tumor budding may prompt a resection in some practices. |
| Intermediate level | 5 - 9 tumor buds | | |
| High level | 10 or more tumor buds | | |
| Micropapillary features | Present | Presence of small and tight clusters of tumor cells within cleft-like spaces | Preferably | Risk for LVI and nodal metastasis, poor prognosis |
| Absent | Absence of small and tight clusters of tumor cells within cleft-like spaces | | |
| Immunohistochemistry for MMR proteins | Intact | Nuclear immunoreactivity | Yes | Loss of MMR leads to workup for LS. |
| Loss | Absence of nuclear immunoreactivity | | |


















