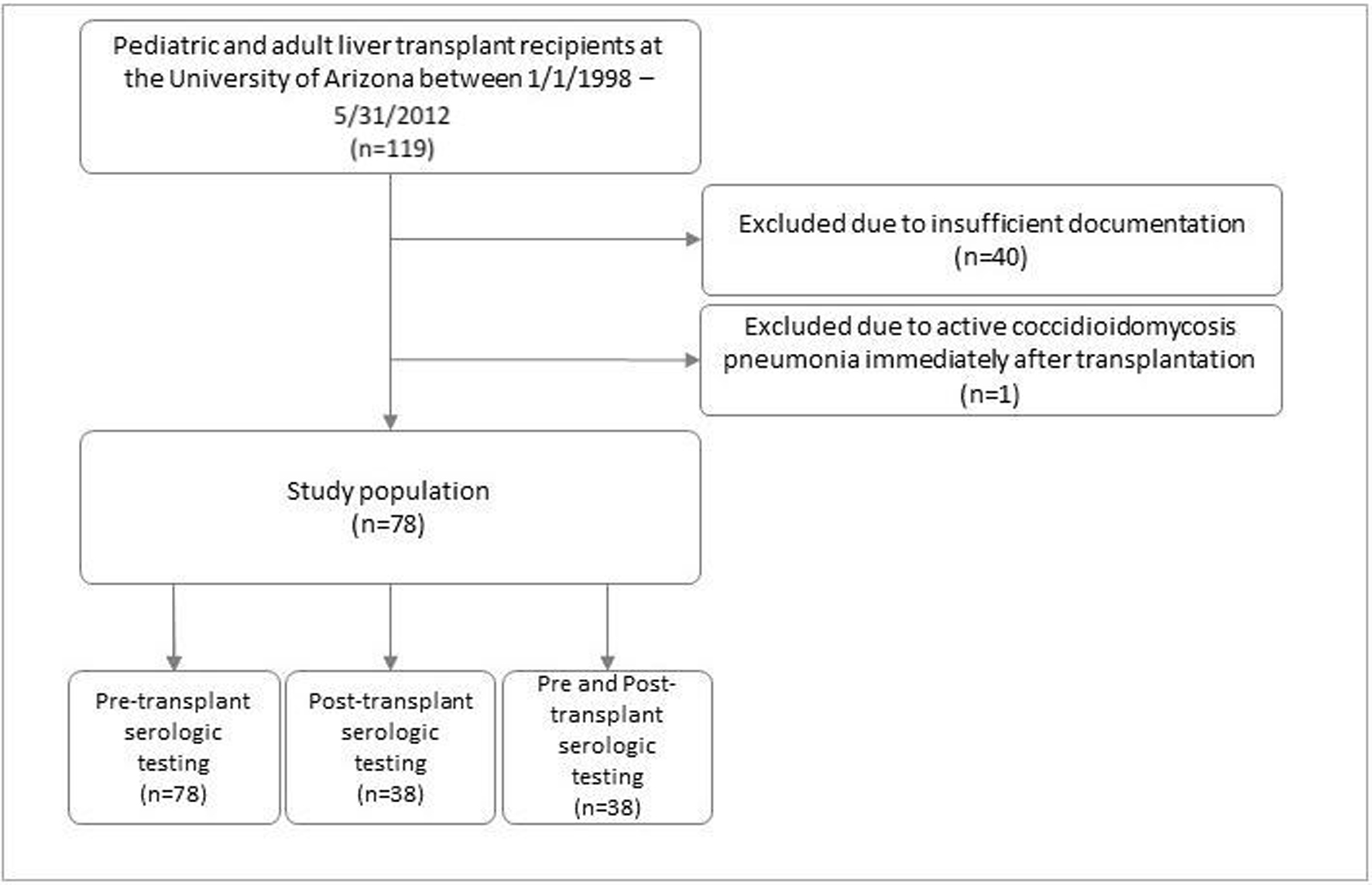
Figure 1. Patient population.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Original Article
Volume 12, Number 3, June 2019, pages 148-156
Efficacy of Low Dose Chemoprophylaxis for Coccidioidomycosis Infection in Liver Transplant Recipients
Figures

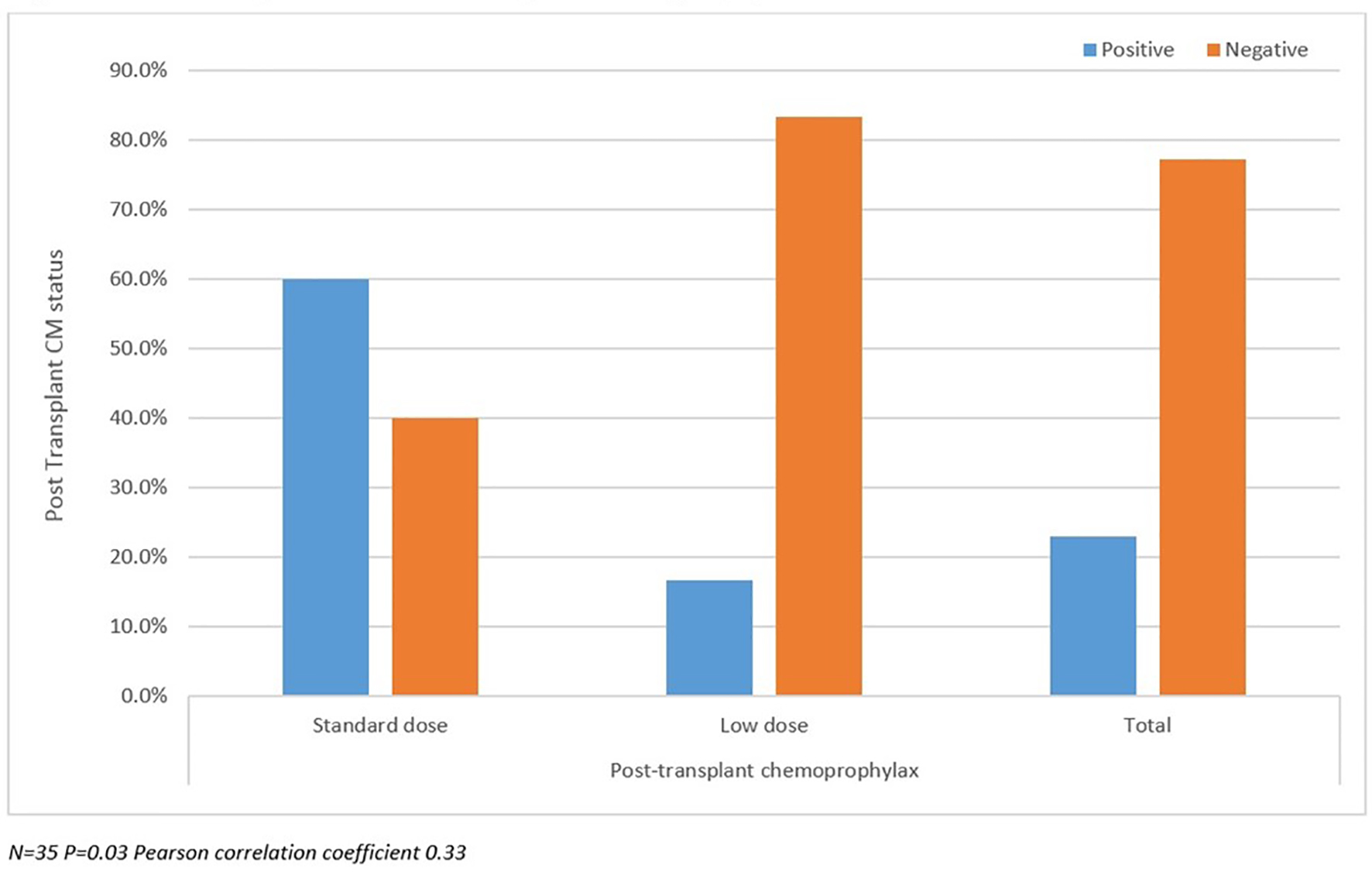
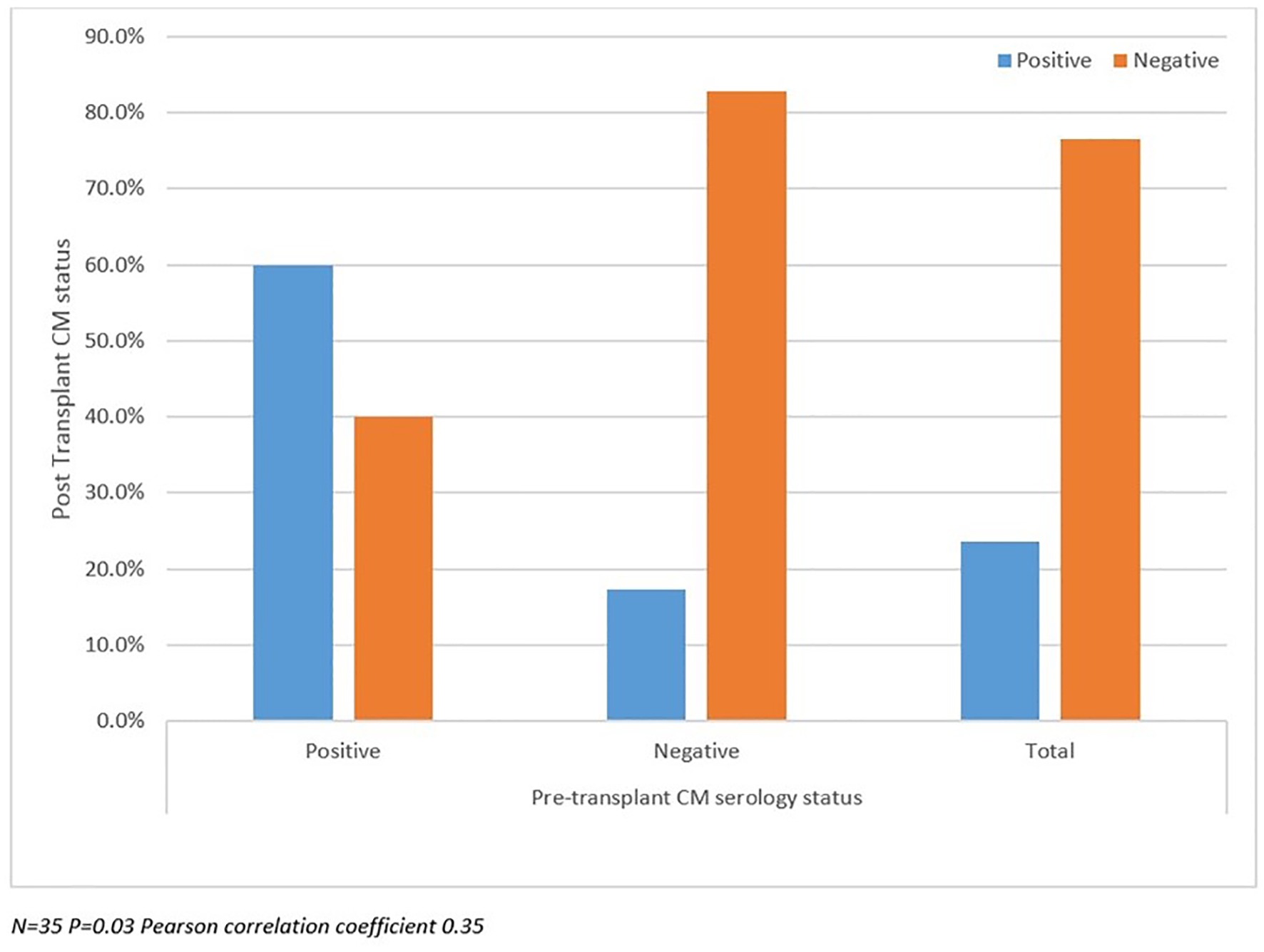
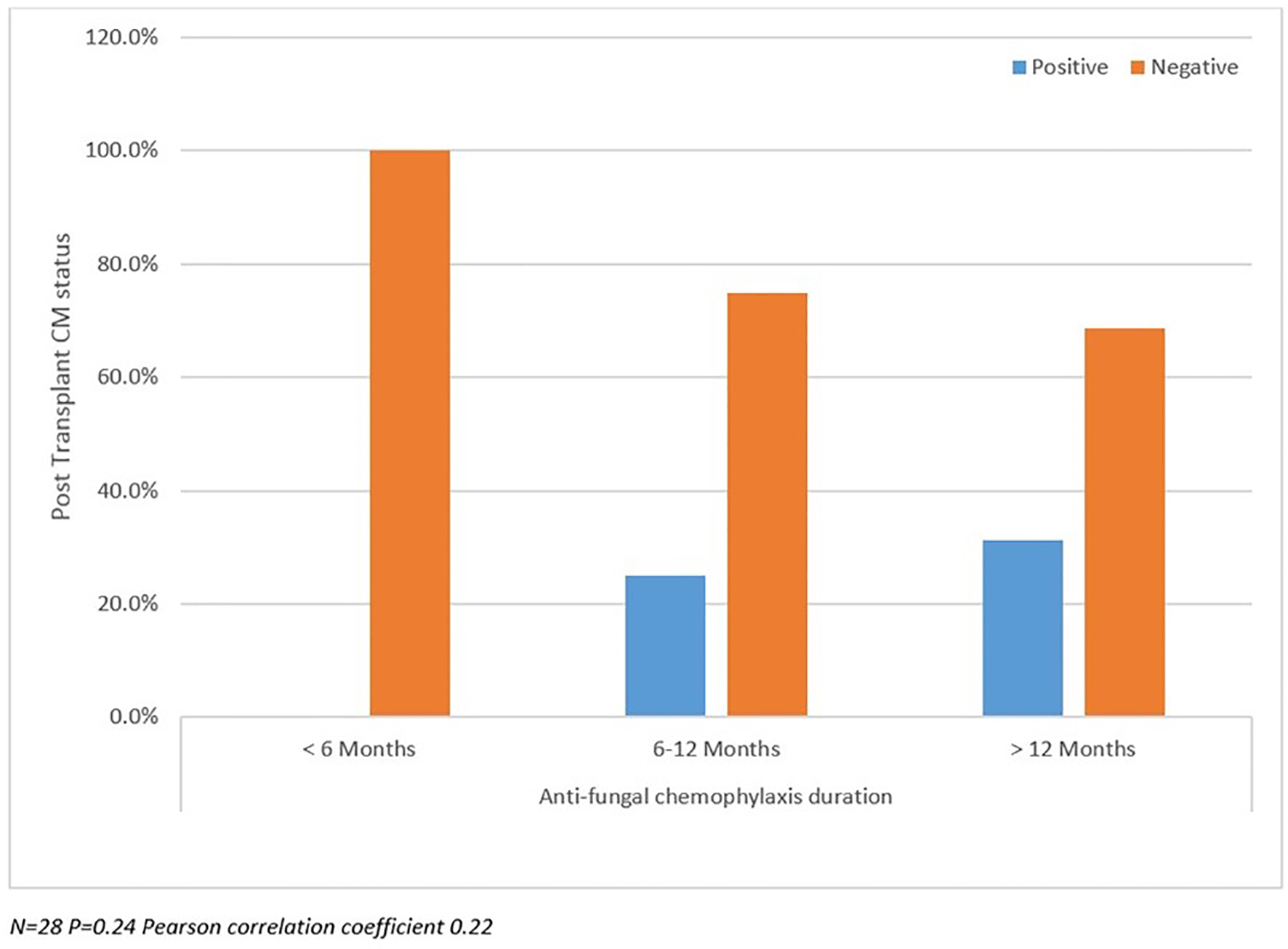
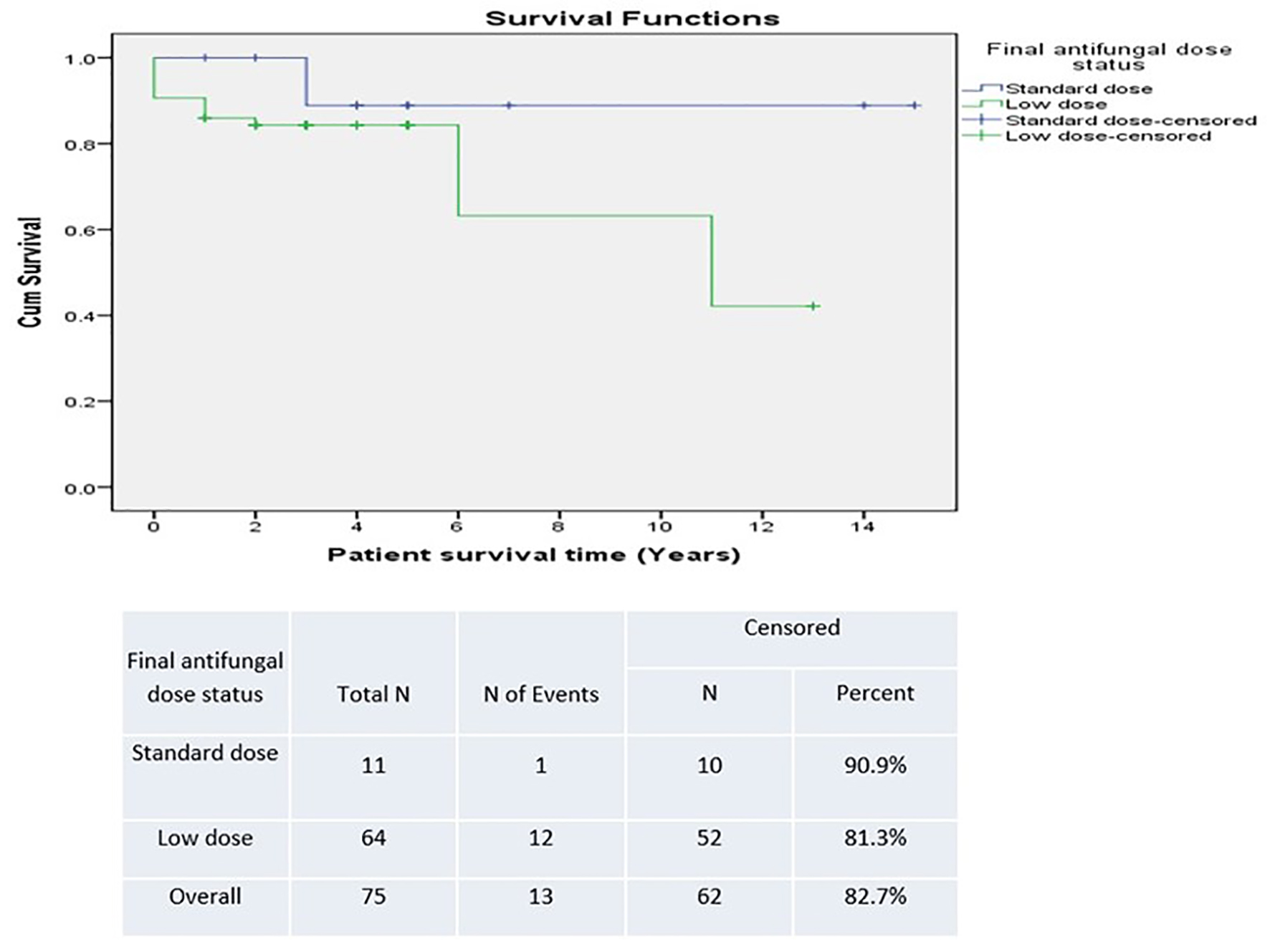
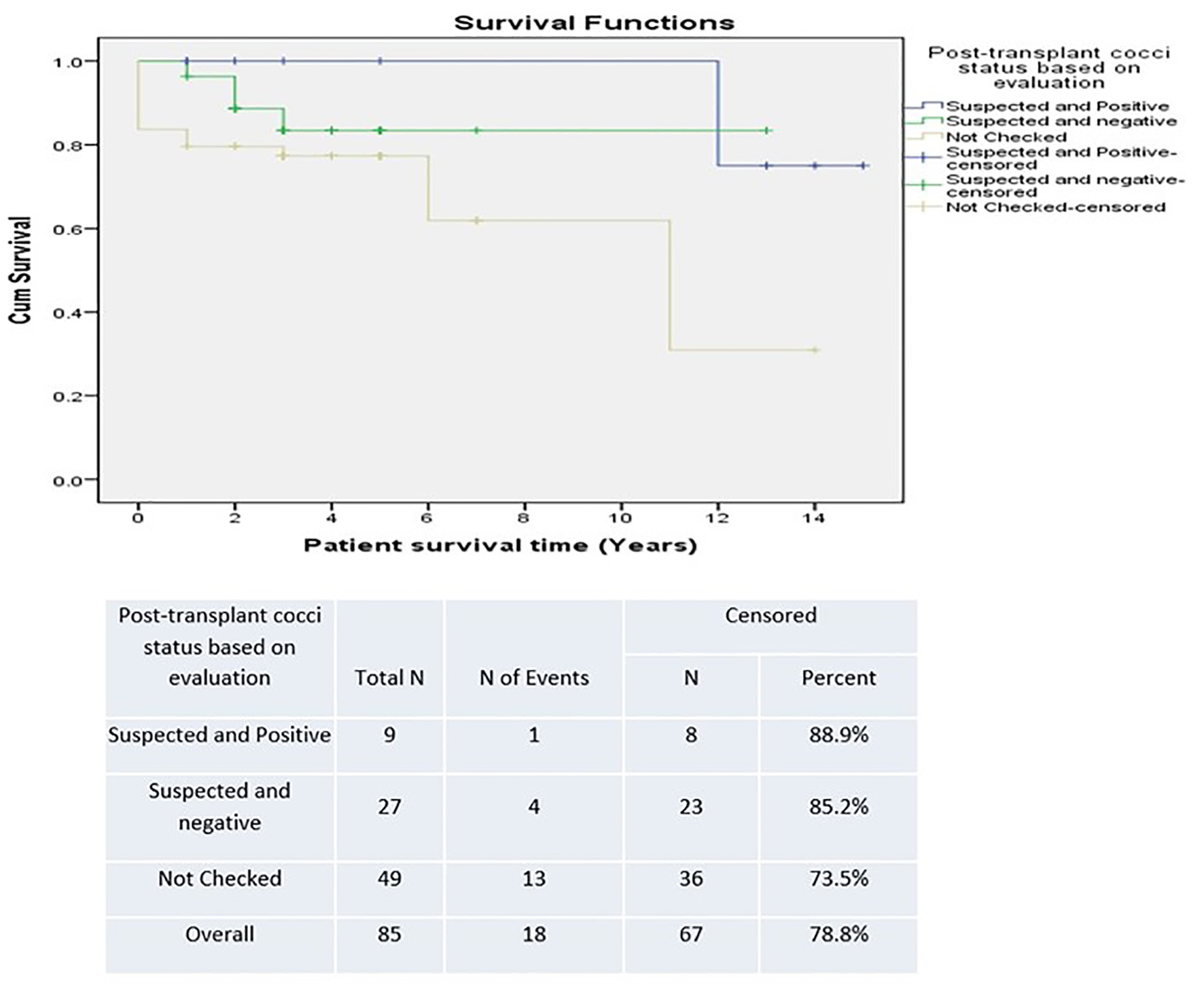
Table
| Baseline characteristics | Total number (n = 78) | Standard dose (n = 11) | Low dose (n = 67) | P-value |
|---|---|---|---|---|
| *Weight and BMI calculated before the time of transplant. **Pre-transplant. BMI: body mass index; ESLD: end-stage liver disease; HCV: hepatitis C virus; HBV: hepatitis B virus; NASH: non-alcoholic steatohepatitis; DM: diabetes mellitus; HTN: hypertension; Ab: antibody. | ||||
| Age in years (range 6 months to 68 years) | ||||
| < 18 years | 33 (42.3%) | 7 (63.6%) | 26 (38.8%) | NS |
| ≥ 18 years | 45 (57.7%) | 4 (36.4%) | 41 (61.2%) | |
| Mean age | 18.9 | 34.3 | NS | |
| Gender | ||||
| Male | 46 (59.0%) | 5 (45.5%) | 41 (61.2%) | NS |
| Female | 32 (41.0%) | 6 (54.5%) | 26 (38.8%) | |
| Race | ||||
| White | 29 (37.2%) | 2 (18.2%) | 27 (40.3%) | NS |
| Non-white | 49 (62.8%) | 9 (81.8%) | 40 (59.7%) | |
| ESLD etiology | ||||
| Alcoholic | 8 (10.3%) | 0 (00.0%) | 8 (11.9%) | NS |
| HCV | 22 (28.2%) | 2 (18.2%) | 20 (29.9%) | |
| HBV | 1 (1.3%) | 0 (0.00%) | 1 (1.5%) | |
| NASH | 9 (11.5%) | 1 (9.1%) | 8 (11.9%) | |
| Other | 38 (48.7%) | 8 (72.7%) | 30 (44.8%) | |
| Weight in pounds, mean* | 70.5 | 136.9 | 0.01 | |
| BMI, mean* | 19.4 | 26.0 | 0.01 | |
| History of DM** | 17 (22.1%) | 1 (10.0%) | 16 (23.9%) | NS |
| History of HTN** | 17 (22.4%) | 3 (30.0%) | 14 (21.2%) | NS |
| History of cancer** | 20 (26.3%) | 3 (30.0%) | 17 (25.8%) | NS |
| Immunosuppression | ||||
| Tacrolimus | 67 (88.2%) | 9 (90.0%) | 58 (87.9%) | NS |
| Prednisone | 58 (76.3%) | 9 (81.8%) | 49 (75.4%) | NS |
| Mycophenolate mofetil | 59 (78.7%) | 7 (70.0%) | 52 (80.0%) | NS |
| Sirolimus | 6 (8.1%) | 1 (10.0%) | 5 (7.8%) | NS |
| Induction therapy | 29 (59.2%) | 1 (33.3%) | 28 (60.9%) | NS |
| Rejection | ||||
| Monoclonal Ab beyond 30 days of transplant | 4 (9.8%) | 0 (0.00%) | 4 (11.1%) | NS |
| Steroid bolus | 30 (73.2%) | 2 (40.0%) | 28 (77.8%) | NS |