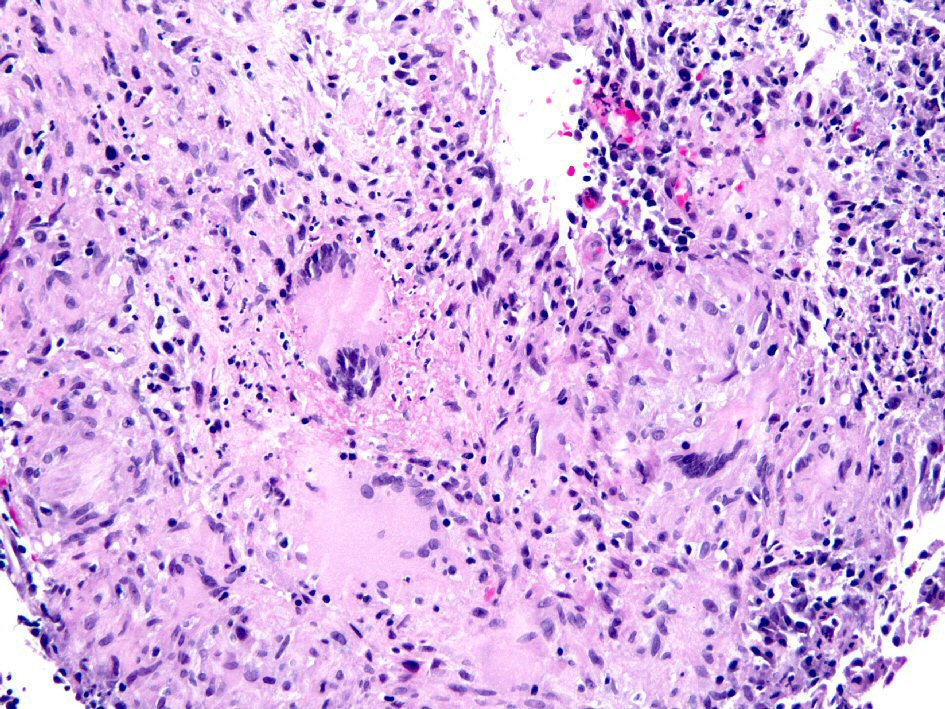
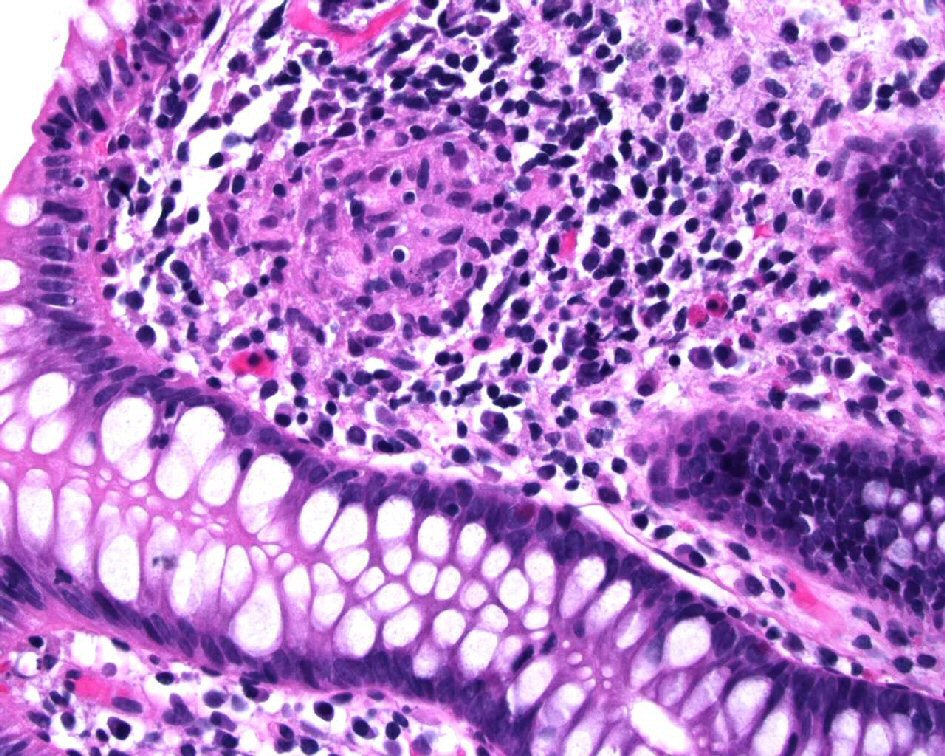
Figure 1. Biopsy from the colon revealed extensive large epithelioid granulomas with central necrosis. Acid-fast mycobacteria were identified by Ziehl-Neelsen stain in colon biopsies (picture not shown).
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Review
Volume 11, Number 3, June 2018, pages 174-188
Anti-Inflammatory Biologics and Anti-Tumoral Immune Therapies-Associated Colitis: A Focused Review of Literature
Figures


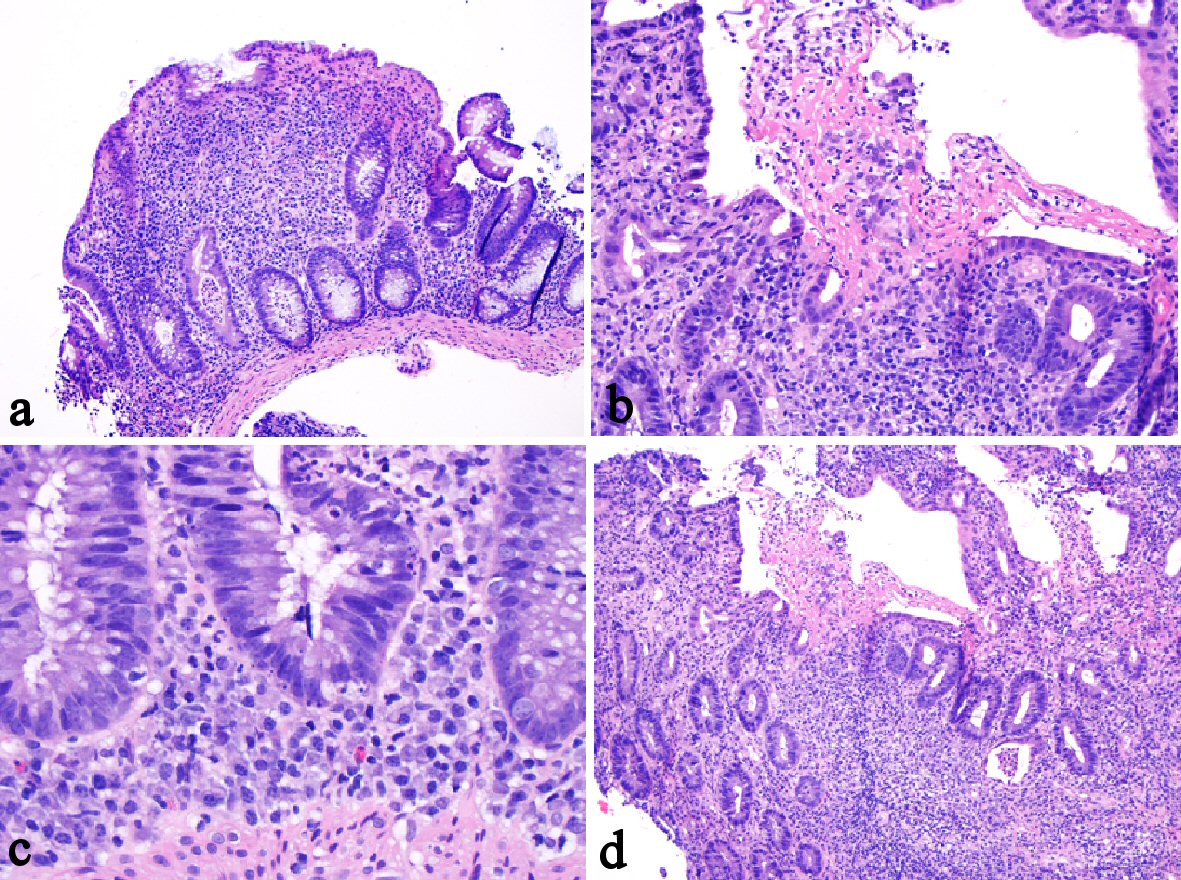
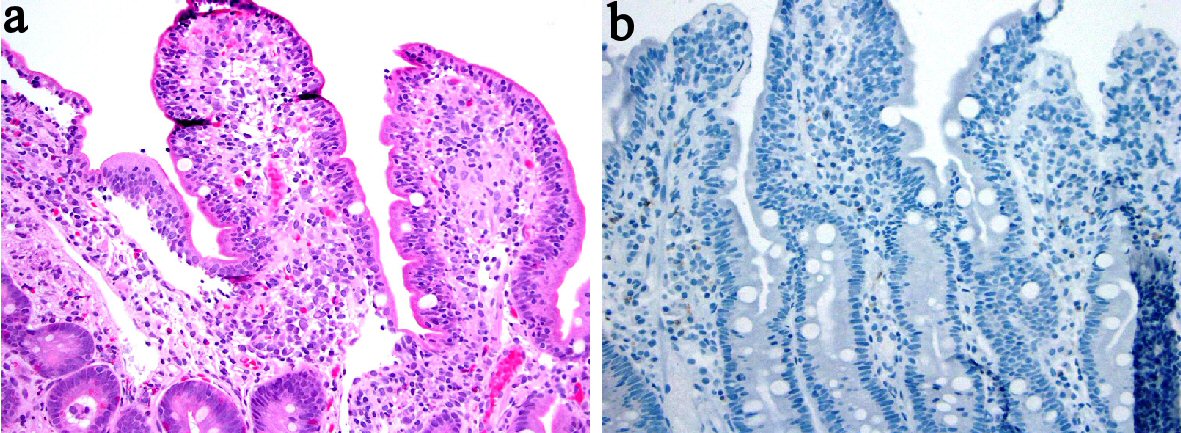
Tables
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|---|
| Colitis | Asymptomatic, pathologic or radiographic findings only | Abdominal pain; mucus or blood in stool | Abdominal pain; fever; change in bowel habits with ileum; peritoneal signs | Life-threatening consequences (e.g. perforation, bleeding, ischemia, necrosis, toxic megacolon) | Death |
| Drug name | Registration name | Structure | Ligands | Molecular weight (kDa) | Half-life (days) | Clinical use | Other features |
|---|---|---|---|---|---|---|---|
| AS: ankylosing spondylitis; CD: Crohn’s disease; IBD: inflammatory bowel disease; JIA: juvenile idiopathic arthritis; PA: psoriatic arthritis; PP: plaque psoriasis; RA: rheumatoid arthritis; TNF: tumor necrosis factor; UC: ulcerative colitis. | |||||||
| Infliximab | Remicade | Chimeric monoclonal antibody | Soluble and transmembrane TNF-α | 150 | 8 - 10 | CD, UC, RA, AS, PA, PP | |
| Adalimumab | Humira | Human monoclonal antibody | Soluble and transmembrane TNF-α | 150 | 10 - 20 | CD, RA, AS, PA, PP, JIA | |
| Golimumab | Simponi | Human monoclonal antibody | Soluble and transmembrane TNF-α | 150 | 7 - 20 | RA, AS, PA | |
| Certolizumab pegol | Cimzia | TNF-specific, PEGylated Fab’ antibody fragment | Soluble and transmembrane TNF-α | About 95 | - 14 | RA, PA, AS, CD | |
| Etanercept | Enbrel | A fusion protein of two TNFR2 receptor extracellular domains and the Fc portion of human IgG | Soluble and transmembrane TNF-α | 150 | 4 | RA, JIA, PA, AS, PP | No efficacy in IBD |
| Agent | Colitis | Histology features | Clinical presentation | Treatment | Clinical outcome | References |
|---|---|---|---|---|---|---|
| CD: Crohn’s disease; JRA: Juvenile rheumatoid arthritis; N/A: information not available; RA: rheumatoid arthritis; TB: tuberculosis; TNF: tumor necrosis factor; UC: ulcerative colitis. | ||||||
| Infliximab | UC (n = 1) | Chronic active colitis with cryptitis, crypt abscesses, architectural distortion, dense lymphoplasmacytic infiltrate | Bloody diarrhea 2 weeks after his fourth infliximab inclusion | Cessation of infliximab; Parental steroids; Mesalamine | Resolved | [6] |
| Apoptotic enteropathy (n = 1) | Architectural distortion, empty appearing lamina propria, cystically dilated crypts with atrophic epithelial lining, scattered apoptosis of basal crypt epithelium | Watery diarrhea | Cessation of infliximab | Diarrhea improved at 1 month following the last dose of infliximab | [8] | |
| Ischemic colitis (n = 13) | N/A | N/A | N/A | 3 died; 9 recovered; 1 without follow-up data | [9] | |
| Intestinal TB | Intestinal and pulmonary TB (n = 1) | N/A | N/A | N/A | [12] | |
| Intestinal, pulmonary, brain TB (n = 1) | Worsening diarrhea | Anti-TB treatment | Died 8 months after diagnosis of TB | [13] | ||
| CMV colitis (n = 1) | Features not well depicted in the text; No pictures from H&E stained sections presented; Pictures from immunostain equivocal for CMV at the most | Developed abdominal discomfort, anorexia, epigastric pain, watery diarrhea at 10 days after the third dose of infliximab | Cessation of infliximab and starting ganciclovir | No recurrence of diarrhea at 30 months of follow-up | [15] | |
| Adalimumab | Ischemic colitis (n = 13) | Ischemic colitis without vasculitis on right hemicolectomy specimen (n = 1) | Developed post-prandial bilious vomiting, right flank and upper quadrant cramping pain and diarrhea 1 week after initiating adalimumab for RA | Right hemicolectomy | Alive after right hemicolectomy | [16] |
| N/A (n = 12) | N/A | N/A | 1 died; 10 recovered; 1 without follow-up data | [9] | ||
| Apoptotic enteropathy (n = 2) | Increase in apoptotic bodies in the duodenum and mild increase in apoptotic bodies in the colon | Diarrhea or abdominal pain | N/A | N/A | [17] | |
| Disseminated TB with mycobacteria detected in feces (n = 1) | N/A | N/A | N/A | N/A | [12] | |
| Indolent T-cell lymphoproliferative disease of the gastrointestinal tract (n = 1) | Active chronic colitis and multiple foci of small lymphocyte infiltrates expanding the lamina propria of the inflamed mucosa without crypt destruction. The T cells are CD8+, TIA-1+, TCRβ-F1+ with TCRG and TCRB gene rearrangement by PCR study | Not mentioned | Cessation of adalimumab | Monoclonal T-cell infiltrate reappeared at the only site of active inflammation | [18] | |
| Certolizumab pegol | Ischemic colitis (n = 3) | N/A | N/A | N/A | 1 recovered; 1 without follow-up data | [9] |
| Etanercept | UC | N/A | Developed diarrhea; No resolution of UC after cessation of etanercept, need anti-IBD standard therapy and/or other anti-TNF-α agent | N/A | N/A | [19] |
| Histology of UC (n = 1) | Developed bloody diarrhea after 28 months on etanercept for JRA | Flaring on adalimumab and infliximab, resolution only after cessation of all anti-TNF agents | Healing UC confirmed by colonoscopy 10 months after cessation of infliximab | [6] | ||
| Histology of UC (n = 3) and one with microgranuloma | N/A | Cessation of etanercept and need anti-IBD with anti-TNF-α agents in two patients | Resolution in 2; 1 without follow-up data | [27] | ||
| CD (n = 9) | Epithelioid granuloma present; Also with upper gastrointestinal tract involvement | N/A | Cessation of etanercept in most cases, need anti-IBD standard therapy and/or other anti-TNF-α agent | 4 with remission; 5 without remission | [26, 27] | |
| Ischemic colitis (n = 7) | N/A | N/A | N/A | 1 died; 6 recovered | [9] | |
| Apoptotic enteropathy (n = 1) | Prominent apoptotic bodies in the duodenum and mild increase in apoptotic bodies in the colon | Diarrhea | N/A | N/A | [17] | |
| Sarcoid-like lesions | N/A | N/A | N/A | N/A | [28] | |
| Case | Age (years) | Gender | Underlying disease | Rituximab dose and duration | Other treatment | Interval between completion to symptoms of colitis | Diagnosis | Histology confirmation | Follow-up | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| N/A: information not available; R-CHOP: rituximab, cyclophosphamide, hydroxydaunomycin, vincristine, prednisolone; R-CVP: rituximab, cyclophosphamide, vincristine, and prednisolone; RTX: rituximab; SLE: systemic lupus erythematosus. | ||||||||||
| 1 | 62 | Female | Marginal zone B cell lymphoma | Several cycles of RTX (2002 - 2005) and four doses in 2005 | None | Not mention (short) | Diffuse colitis with pneumatosis | Fulminant colitis on subcolectomy specimen | Symptom recurred 5 years later after receiving a second course of four cycles of RTX, developed biopsy proven colitis. | [63] |
| 2 | 67 | Male | Relapse of follicular lymphoma | Four cycles of fludarabine, cyclophosphamide with rituximab and then maintenance therapy with RTX at a dose of 375 mg/m2 every 3 months for a total of six cycles | None | 2 months after his sixth cycle of RTX | Diffuse pancolitis | Confirmed by histology on subtotal colectomy | Patient died of recurrence of bacterial pneumonia 4 months after surgery. | [68] |
| 3 | 26 | Female | Non-Hodgkin lymphoma | N/A | N/A | N/A | Fulminant colitis | Confirmed by histology on colectomy | N/A | [69] |
| 4 | 45 | Female | Grave’s disease (clinical trial) | After the second infusion with weekly dose of 375 mg/m2 | None | During treatment | Ulcerative colitis | Confirmed by sigmoidoscopy and biopsy | Mesalamine induced remission on day 650. | [67] |
| 5 | 4 | Male | Refractory nephrotic syndrome | 4-week course of rituximab at a dose of 375 mg/m2 | FK-506 | 6 weeks after RTX therapy | Severe ulcerative colitis | Confirmed by colonoscopy and biopsy | Corticosteroid induced remission with endoscopic and histologic healing. | [66] |
| 6 | 34 | Unknown | Bullous SLE | After three infusions at a dose of 375 mg/m2 | N/A | During treatment | Ulcerative colitis | Confirmed by colonoscopy and biopsy | Episode of acute appendicitis with appendectomy. Later developed UC. Symptoms resolved upon RTX discontinuation. | [70] |
| 7 | 38 | Female | Refractory seronegative rheumatoid arthritis | N/A | None | 13 weeks | Ulcerative colitis | Confirmed by colonoscopy and biopsy | Corticosteroid and 5-ASA induced remission; follow-up biopsy one year after RTX cessation showed restoration of B cells in the colonic mucosa and resolution of colitis. | [64] |
| 8 | 80 | Female | Small lymphocytic lymphoma | RTX 3-month maintenance | R-CVP | 3 months | Crohn’s disease | Confirmed by colonoscopy/biopsy and resection histology | Surgical resection | [62] |
| 9 | 74 | Male | Grade 2 follicular lymphoma | RTX 3-month maintenance | R-CHOP | 22 months | Crohn’s disease | Confirmed by colonoscopy and biopsy inflammation and small granulomata | Treated with hydrocortisone | [62] |
| Case | Clinical | Imaging study | Colonoscopy | Lab | Biopsy finding | Histology on colectomy | IHC | Final diagnosis | Reference |
|---|---|---|---|---|---|---|---|---|---|
| CMV: cytomegalovirus; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; FGD: fluorodeoxyglucose; Hb: hemoglobin; N/A: information not available; N/D: not done; PET-CT: positron emission tomography/computed tomography; RA: rheumatoid arthritis; RTX: rituximab; SLE: systemic lupus erythematosus. | |||||||||
| 1 | Severe abdominal pain and diarrhea | N/A | Diffuse severe colitis on sigmoidoscopy | N/D | N/A | Diffuse colitis with pneumatosis in colectomy specimen | N/D | Fulminant colitis | [63] |
| 2 | 2-week history of fever, cough, shortness of breath, and watery diarrhea. On the sixth day of admission the patient developed severe bloody diarrhea (6 - 7 bowel movements/day) | A CT scan revealed diffuse thickening of the entire colon with pericolic stranding adjacent to cecum and ascending colon. | Flexible sigmoidoscopy revealed severe confluent inflammation extending from the anorectal junction to the proximal limit of viewing at 30 cm | CBC neutrophils was 4.9 × 109/L; Stool specimens were negative for Salmonella, Shigella, Campylobacter, parasites, and Clostridium difficile toxin A and B | Biopsies from the rectal and colonic mucosa revealed almost complete dropout of tubules and almost complete loss of surface epithelium without any pseudomembranes. The few residual crypts showed marked depletion of mucin and extensive apoptosis. Immunohistochemistry for CMV was negative. | Total colectomy 2 weeks after the initial onset of bloody diarrhea because of failure to respond to hydrocortisone therapy. Histology of colectomy showed diffuse pancolitis and terminal ileitis. | Lack of CD20+ cells; Normal number to a moderate increase of CD3+ cells; Moderate excess of enlarged macrophages | Severe apoptotic enterocolopathy/RTX-induced immunodysregulatory ileocolitis | [68] |
| 3 | N/A | N/A | N/A | N/A | N/A | Fulminant colitis | N/A | Fulminant colitis | [69] |
| 4 | Developed bloody diarrhea up to 20 stools per day | N/D | Sigmoidoscopy revealed colitis | N/D | Colonic biopsy taken 67days after initiation of RTX: inflammation, irregular crypts, and crypt abscesses | N/D | Biopsy taken 67 days after initiation of RTX: absence of CD20+ cells | Ulcerative colitis | [67] |
| 5 | Developed crampy abdominal pain, bloody diarrhea with 20 stools per day, weight loss, intermittent fevers | Abdominal ultrasound revealed severe pancolitis with wall thickening up to 6 mm. | Severe pancolitis with deep ulcers | Blood, urine, stool cultures negative. Clostridium difficile assays negative. Torovirus detected in the stool by electron microscopy. | Cryptitis with mixed inflammation in the lamina propria. No granulomata were identified. | N/D | Biopsy: absence of CD20+ cells | Severe ulcerative colitis | [66] |
| 6 | Profuse watery diarrhea | N/A | Erythematous mucosa (colitis) | N/A | Important infiltrates composed of mainly CD8+ T lymphocytes | N/D | Biopsy: primarily CD8+ T lymphocytes | Ulcerative colitis developed after initial episode of acute appendicitis | [70] |
| 7 | Bloody diarrhea developed 7 weeks after completion of biweekly at a dose of 1 g/2 weeks | N/D | Moderately severe pancolitis | N/D | Biopsy at 13 weeks: Goblet cell depletion, active and chronic inflammation with crypt abscess | N/D | Biopsy at 13 weeks: absence of CD20+ cell in the colonic biopsies | Ulcerative colitis | [64] |
| 8 | Crohn’s disease | [62] | |||||||
| Two episodes of fever and diarrhea | N/D | Non-specific transverse colon ulcer, normal ileum (at the first episode) | Clostridium difficile was positive for the first episode. | No viral inclusions, granulomas, or changes of chronicity | Treated with metronidazole but had ongoing exacerbations of abdominal pain and diarrhea. | N/D | |||
| A few month later, patient presented with abdominal pain, rectal bleeding | PET-CT revealed FDG-avid disease in her ileum when the patient had abdominal pain. | Ulcer at ileocolic anastomosis and an ulcer at the hepatic flexure and left colon | Fecal calprotectin 5,403 mg/kg | Patchy active inflammation with ulceration and multiple small granuloma, some with multinucleated giant cells | Patient underwent resection 3 month later because of the development of inflammatory mass in the right colon despite budesonide therapy. | N/D | |||
| 9 | Crohn’s disease | [62] | |||||||
| 5-week history of diarrhea, abdominal pain, fevers, and 8 kg of weight loss | Distal ileal wall thickening | Ileal inflammation, linear ulceration, but normal colonic mucosa | Anemia (Hb at 116 g/L); ESR of 74 mm/h; CRP of 87 mg/L; Fecal calprotectin of 3,226 mg/kg | N/A | N/D | N/D | |||
| Re-presented 3 weeks later with recurrent fevers and worsening abdominal pain despite budesonide therapy | PET-CT revealed transmural update and thickening in the mid to distal small bowel. | Ileal inflammation, linear ulceration for at least 15 cm very easy contact bleeding, but normal colonic mucosa | N/D | Active ileitis with ulceration; Focal active colitis with ulceration and a single poorly formed granuloma | N/D | N/D | |||